Abstract
Purpose of Review
Malaria parasites continue to demonstrate their ability to evolve drug resistance, underscoring the need to maintain a long-term program of antimalarial drug development. The parasite mitochondrion is an essential organelle in every lifecycle stage. In the parasite, there are ~ 300 proteins encoded on the nuclear genome destined for the mitochondrion (around 6% of the genome), plus 3 protein genes present on the 6-kb mitochondrial genome. Many of these gene products compose pathways that are critical for the mitochondrion and the mitochondrial contribution to the parasite. The mitochondrial electron transport chain (mtETC) is essential for the parasite and has been validated as an antimalarial drug target. Another mitochondrially located target is the parasite dihydroorotate dehydrogenase (DHODH), which is involved in the essential pyrimidine biosynthesis pathway. In this review, we will summarize recent advancements in drug development targeting the mtETC and DHODH. We will also discuss other pathways within the mitochondrion that hold promise for future exploitation in the search for additional antimalarial drug targets.
Recent Findings
Recent drug development efforts have advanced two compounds into clinical evaluation, ELQ-300 and DSM265, targeting the mtETC and DHODH, respectively. These compounds are very potent against malaria parasites at multiple lifecycle stages and have shown good pharmacological properties such as long half-lives and metabolic stability. However, progress toward the development of antimalarial compounds against other mitochondrial functions has been very limited.
Summary
New drugs that target the mtETC and DHODH are likely to reach the clinic in the near future. Additional studies are required to verify the essentiality of other mitochondrial pathways in malaria parasites and validate novel antimalarial drug targets needed to help ensure the continuing future development of new antimalarial drugs.


Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Martin WF, Neukirchen S, Zimorski V, Gould SB, Sousa FL. Energy for two: new archaeal lineages and the origin of mitochondria. BioEssays. 2016;38(9):850–6. https://doi.org/10.1002/bies.201600089.
Pittis AA, Gabaldon T. Late acquisition of mitochondria by a host with chimaeric prokaryotic ancestry. Nature. 2016;531(7592):101–4. https://doi.org/10.1038/nature16941.
Gray MW. The pre-endosymbiont hypothesis: a new perspective on the origin and evolution of mitochondria. Cold Spring Harb Perspect Biol. 2014;6(3). doi:https://doi.org/10.1101/cshperspect.a016097.
Adl SM, Leander BS, Simpson AG, Archibald JM, Anderson OR, Bass D, et al. Diversity, nomenclature, and taxonomy of protists. Syst Biol. 2007;56(4):684–9. https://doi.org/10.1080/10635150701494127.
Poinar G Jr. Plasmodium dominicana n. sp. (Plasmodiidae: Haemospororida) from Tertiary Dominican amber. Syst Parasitol. 2005;61(1):47–52. https://doi.org/10.1007/s11230-004-6354-6.
WHO: World Malaria Report. 2016. http://www.who.int/malaria/publications/world-malaria-report-2016/report/en/.
Vaidya AB, Arasu P. Tandemly arranged gene clusters of malarial parasites that are highly conserved and transcribed. Mol Biochem Parasitol. 1987;22(2–3):249–57.
Vaidya AB, Akella R, Suplick K. Sequences similar to genes for two mitochondrial proteins and portions of ribosomal RNA in tandemly arrayed 6-kilobase-pair DNA of a malarial parasite. Mol Biochem Parasitol. 1989;35(2):97–107.
Vaidya AB. Mitochondrial and plastid functions as antimalarial drug targets. Curr Drug Targets Infect Disord. 2004;4(1):11–23.
Vaidya AB, Mather MWA. Post-genomic view of the mitochondrion in malaria parasites. Curr Top Microbiol Immunol. 2005;295:233–50.
Mather MW, Vaidya AB. Mitochondria in malaria and related parasites: ancient, diverse and streamlined. J Bioenerg Biomembr. 2008;40(5):425–33. https://doi.org/10.1007/s10863-008-9176-4.
Vaidya AB, Mather MW. Mitochondrial evolution and functions in malaria parasites. Annu Rev Microbiol. 2009;63:249–67. https://doi.org/10.1146/annurev.micro.091208.073424.
Sheiner L, Vaidya AB, McFadden GI. The metabolic roles of the endosymbiotic organelles of Toxoplasma and Plasmodium spp. Curr Opin Microbiol. 2013;16(4):452–8. https://doi.org/10.1016/j.mib.2013.07.003.
Hikosaka K, Kita K, Tanabe K. Diversity of mitochondrial genome structure in the phylum Apicomplexa. Mol Biochem Parasitol. 2013;188(1):26–33. https://doi.org/10.1016/j.molbiopara.2013.02.006.
Jacot D, Waller RF, Soldati-Favre D, MacPherson DA, MacRae JI. Apicomplexan energy metabolism: carbon source promiscuity and the quiescence hyperbole. Trends Parasitol. 2016;32(1):56–70. https://doi.org/10.1016/j.pt.2015.09.001.
Sherman IW. Biochemistry of Plasmodium (malarial parasites). Microbiol Rev. 1979;43(4):453–95.
Balabaskaran Nina P, Morrisey JM, Ganesan SM, Ke H, Pershing AM, Mather MW, et al. ATP synthase complex of Plasmodium falciparum: dimeric assembly in mitochondrial membranes and resistance to genetic disruption. J Biol Chem. 2011;286(48):41312–22. https://doi.org/10.1074/jbc.M111.290973.
Sturm A, Mollard V, Cozijnsen A, Goodman CD, McFadden GI. Mitochondrial ATP synthase is dispensable in blood-stage Plasmodium berghei rodent malaria but essential in the mosquito phase. Proc Natl Acad Sci U S A. 2015;112(33):10216–23. https://doi.org/10.1073/pnas.1423959112.
Balabaskaran Nina P, Dudkina NV, Kane LA, van Eyk JE, Boekema EJ, Mather MW, et al. Highly divergent mitochondrial ATP synthase complexes in Tetrahymena thermophila. PLoS Biol. 2010;8(7):e1000418. https://doi.org/10.1371/journal.pbio.1000418.
Gutteridge WE, Dave D, Richards WH. Conversion of dihydroorotate to orotate in parasitic protozoa. Biochim Biophys Acta. 1979;582(3):390–401.
Painter HJ, Morrisey JM, Mather MW, Vaidya AB. Specific role of mitochondrial electron transport in blood-stage Plasmodium falciparum. Nature. 2007;446(7131):88–91. https://doi.org/10.1038/nature05572.
Phillips MA, Rathod PK. Plasmodium dihydroorotate dehydrogenase: a promising target for novel anti-malarial chemotherapy. Infect Disord Drug Targets. 2010;10(3):226–39.
Rodrigues T, Lopes F, Moreira R. Inhibitors of the mitochondrial electron transport chain and de novo pyrimidine biosynthesis as antimalarials: the present status. Curr Med Chem. 2010;17(10):929–56.
Nixon GL, Pidathala C, Shone AE, Antoine T, Fisher N, O'Neill PM, et al. Targeting the mitochondrial electron transport chain of Plasmodium falciparum: new strategies towards the development of improved antimalarials for the elimination era. Future Med Chem. 2013;5(13):1573–91. https://doi.org/10.4155/fmc.13.121.
Stocks PA, Barton V, Antoine T, Biagini GA, Ward SA, O'Neill PM. Novel inhibitors of the Plasmodium falciparum electron transport chain. Parasitology. 2014;141(1):50–65. https://doi.org/10.1017/S0031182013001571.
Mitchell P. The protonmotive Q cycle: a general formulation. FEBS Lett. 1975;59(2):137–9.
Vaidya AB, Lashgari MS, Pologe LG, Morrisey J. Structural features of Plasmodium cytochrome b that may underlie susceptibility to 8-aminoquinolines and hydroxynaphthoquinones. Mol Biochem Parasitol. 1993;58(1):33–42.
Fieser LF, Heymann H. Naphthoquinone antimalarials; relative antirespiratory activities (Plasmodium lophurae). J Biol Chem. 1948;176(3):1363–70.
Fry M, Pudney M. Site of action of the antimalarial hydroxynaphthoquinone, 2-[trans-4-(4′-chlorophenyl) cyclohexyl]-3-hydroxy-1,4-naphthoquinone (566C80). Biochem Pharmacol. 1992;43(7):1545–53.
Chiodini PL, Conlon CP, Hutchinson DB, Farquhar JA, Hall AP, Peto TE, et al. Evaluation of atovaquone in the treatment of patients with uncomplicated Plasmodium falciparum malaria. J Antimicrob Chemother. 1995;36(6):1073–8.
Looareesuwan S, Viravan C, Webster HK, Kyle DE, Hutchinson DB, Canfield CJ. Clinical studies of atovaquone, alone or in combination with other antimalarial drugs, for treatment of acute uncomplicated malaria in Thailand. Am J Trop Med Hyg. 1996;54(1):62–6.
Canfield CJ, Pudney M, Gutteridge WE. Interactions of atovaquone with other antimalarial drugs against Plasmodium falciparum in vitro. Exp Parasitol. 1995;80(3):373–81. https://doi.org/10.1006/expr.1995.1049.
Markley LD, Van Heertum JC, Doorenbos HE. Antimalarial activity of clopidol, 3,5-dichloro-2,6-dimethyl-4-pyridinol, and its esters, carbonates, and sulfonates. J Med Chem. 1972;15(11):1188–9.
Xiang H, McSurdy-Freed J, Moorthy GS, Hugger E, Bambal R, Han C, et al. Preclinical drug metabolism and pharmacokinetic evaluation of GW844520, a novel anti-malarial mitochondrial electron transport inhibitor. J Pharm Sci. 2006;95(12):2657–72. https://doi.org/10.1002/jps.20681.
Bueno JM, Herreros E, Angulo-Barturen I, Ferrer S, Fiandor JM, Gamo FJ, et al. Exploration of 4(1H)-pyridones as a novel family of potent antimalarial inhibitors of the plasmodial cytochrome bc1. Future Med Chem. 2012;4(18):2311–23. https://doi.org/10.4155/fmc.12.177.
Yeates CL, Batchelor JF, Capon EC, Cheesman NJ, Fry M, Hudson AT, et al. Synthesis and structure-activity relationships of 4-pyridones as potential antimalarials. J Med Chem. 2008;51(9):2845–52. https://doi.org/10.1021/jm0705760.
Capper MJ, O'Neill PM, Fisher N, Strange RW, Moss D, Ward SA, et al. Antimalarial 4(1H)-pyridones bind to the qi site of cytochrome bc1. Proc Natl Acad Sci U S A. 2015;112(3):755–60. https://doi.org/10.1073/pnas.1416611112.
Monastyrskyi A, Kyle DE, Manetsch R. 4(1H)-pyridone and 4(1H)-quinolone derivatives as antimalarials with erythrocytic, exoerythrocytic, and transmission blocking activities. Curr Top Med Chem. 2014;14(14):1693–705.
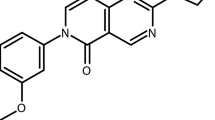
•• Nilsen A, LaCrue AN, White KL, Forquer IP, Cross RM, Marfurt J, et al. Quinolone-3-diarylethers: a new class of antimalarial drug. Sci Transl Med. 2013;5(177):177ra37. https://doi.org/10.1126/scitranslmed.3005029. The preclinical candidate ELQ-300 is a novel bc 1 complex inhibitor with high potency and multi-stage antimalarial activity.
Nilsen A, Miley GP, Forquer IP, Mather MW, Katneni K, Li Y, et al. Discovery, synthesis, and optimization of antimalarial 4(1H)-quinolone-3-diarylethers. J Med Chem. 2014;57(9):3818–34. https://doi.org/10.1021/jm500147k.
Miley GP, Pou S, Winter R, Nilsen A, Li Y, Kelly JX, et al. ELQ-300 prodrugs for enhanced delivery and single-dose cure of malaria. Antimicrob Agents Chemother. 2015;59(9):5555–60. https://doi.org/10.1128/AAC.01183-15.
Stickles AM, de Almeida MJ, Morrisey JM, Sheridan KA, Forquer IP, Nilsen A, et al. Subtle changes in endochin-like quinolone structure alter the site of inhibition within the cytochrome bc1 complex of Plasmodium falciparum. Antimicrob Agents Chemother. 2015;59(4):1977–82. https://doi.org/10.1128/AAC.04149-14.
Stickles AM, Smilkstein MJ, Morrisey JM, Li Y, Forquer IP, Kelly JX, et al. Atovaquone and ELQ-300 combination therapy as a novel dual-site cytochrome bc1 inhibition strategy for malaria. Antimicrob Agents Chemother. 2016;60(8):4853–9. https://doi.org/10.1128/AAC.00791-16.
Biagini GA, Fisher N, Berry N, Stocks PA, Meunier B, Williams DP, et al. Acridinediones: selective and potent inhibitors of the malaria parasite mitochondrial bc1 complex. Mol Pharmacol. 2008;73(5):1347–55. https://doi.org/10.1124/mol.108.045120.
Winter RW, Kelly JX, Smilkstein MJ, Dodean R, Bagby GC, Rathbun RK, et al. Evaluation and lead optimization of anti-malarial acridones. Exp Parasitol. 2006;114(1):47–56. https://doi.org/10.1016/j.exppara.2006.03.014.
Kelly JX, Smilkstein MJ, Cooper RA, Lane KD, Johnson RA, Janowsky A, et al. Design, synthesis, and evaluation of 10-N-substituted acridones as novel chemosensitizers in Plasmodium falciparum. Antimicrob Agents Chemother. 2007;51(11):4133–40. https://doi.org/10.1128/AAC.00669-07.
Kelly JX, Smilkstein MJ, Brun R, Wittlin S, Cooper RA, Lane KD, et al. Discovery of dual function acridones as a new antimalarial chemotype. Nature. 2009;459(7244):270–3. https://doi.org/10.1038/nature07937.
Cross RM, Maignan JR, Mutka TS, Luong L, Sargent J, Kyle DE, et al. Optimization of 1,2,3,4-tetrahydroacridin-9(10H)-ones as antimalarials utilizing structure-activity and structure-property relationships. J Med Chem. 2011;54(13):4399–426. https://doi.org/10.1021/jm200015a.
Valdes AF. Acridine and acridinones: old and new structures with antimalarial activity. Open Med Chem J. 2011;5:11–20. https://doi.org/10.2174/1874104501105010011.
Vyas VK, Ghate M. Recent developments in the medicinal chemistry and therapeutic potential of dihydroorotate dehydrogenase (DHODH) inhibitors. Mini Rev Med Chem. 2011;11(12):1039–55.
Baldwin J, Michnoff CH, Malmquist NA, White J, Roth MG, Rathod PK, et al. High-throughput screening for potent and selective inhibitors of Plasmodium falciparum dihydroorotate dehydrogenase. J Biol Chem. 2005;280(23):21847–53. https://doi.org/10.1074/jbc.M501100200.
Phillips MA, Gujjar R, Malmquist NA, White J, El Mazouni F, Baldwin J, et al. Triazolopyrimidine-based dihydroorotate dehydrogenase inhibitors with potent and selective activity against the malaria parasite Plasmodium falciparum. J Med Chem. 2008;51(12):3649–53. https://doi.org/10.1021/jm8001026.
Gujjar R, Marwaha A, El Mazouni F, White J, White KL, Creason S, et al. Identification of a metabolically stable triazolopyrimidine-based dihydroorotate dehydrogenase inhibitor with antimalarial activity in mice. J Med Chem. 2009;52(7):1864–72. https://doi.org/10.1021/jm801343r.
Gujjar R, El Mazouni F, White KL, White J, Creason S, Shackleford DM, et al. Lead optimization of aryl and aralkyl amine-based triazolopyrimidine inhibitors of Plasmodium falciparum dihydroorotate dehydrogenase with antimalarial activity in mice. J Med Chem. 2011;54(11):3935–49. https://doi.org/10.1021/jm200265b.
Coteron JM, Marco M, Esquivias J, Deng X, White KL, White J, et al. Structure-guided lead optimization of triazolopyrimidine-ring substituents identifies potent Plasmodium falciparum dihydroorotate dehydrogenase inhibitors with clinical candidate potential. J Med Chem. 2011;54(15):5540–61. https://doi.org/10.1021/jm200592f.
•• Phillips MA, Lotharius J, Marsh K, White J, Dayan A, White KL, et al. A long-duration dihydroorotate dehydrogenase inhibitor (DSM265) for prevention and treatment of malaria. Sci Transl Med. 2015;7(296):296ra111. https://doi.org/10.1126/scitranslmed.aaa6645. DSM265 is the first PfDHODH inhibitor to reach clinical development. It is highly effective against malaria parasites at multiple stages.
Pavadai E, El Mazouni F, Wittlin S, de Kock C, Phillips MA, Chibale K. Identification of new human malaria parasite Plasmodium falciparum dihydroorotate dehydrogenase inhibitors by pharmacophore and structure-based virtual screening. J Chem Inf Model. 2016;56(3):548–62. https://doi.org/10.1021/acs.jcim.5b00680.
Kokkonda S, Deng X, White KL, Coteron JM, Marco M, de Las Heras L, et al. Tetrahydro-2-naphthyl and 2-indanyl triazolopyrimidines targeting Plasmodium falciparum dihydroorotate dehydrogenase display potent and selective antimalarial activity. J Med Chem. 2016;59(11):5416–31. https://doi.org/10.1021/acs.jmedchem.6b00275.
Patel V, Booker M, Kramer M, Ross L, Celatka CA, Kennedy LM, et al. Identification and characterization of small molecule inhibitors of Plasmodium falciparum dihydroorotate dehydrogenase. J Biol Chem. 2008;283(50):35078–85. https://doi.org/10.1074/jbc.M804990200.
Booker ML, Bastos CM, Kramer ML, Barker RH Jr, Skerlj R, Sidhu AB, et al. Novel inhibitors of Plasmodium falciparum dihydroorotate dehydrogenase with anti-malarial activity in the mouse model. J Biol Chem. 2010;285(43):33054–64. https://doi.org/10.1074/jbc.M110.162081.
Skerlj RT, Bastos CM, Booker ML, Kramer ML, Barker RH, Jr., Celatka CA et al. Optimization of potent inhibitors of P. falciparum dihydroorotate dehydrogenase for the treatment of malaria. ACS Med Chem Lett 2011;2(9):708–713. doi:https://doi.org/10.1021/ml200143c.
Deng X, Gujjar R, El Mazouni F, Kaminsky W, Malmquist NA, Goldsmith EJ, et al. Structural plasticity of malaria dihydroorotate dehydrogenase allows selective binding of diverse chemical scaffolds. J Biol Chem. 2009;284(39):26999–7009. https://doi.org/10.1074/jbc.M109.028589.
Xu M, Zhu J, Diao Y, Zhou H, Ren X, Sun D, et al. Novel selective and potent inhibitors of malaria parasite dihydroorotate dehydrogenase: discovery and optimization of dihydrothiophenone derivatives. J Med Chem. 2013;56(20):7911–24. https://doi.org/10.1021/jm400938g.
Nam TG, McNamara CW, Bopp S, Dharia NV, Meister S, Bonamy GM, et al. A chemical genomic analysis of decoquinate, a Plasmodium falciparum cytochrome b inhibitor. ACS Chem Biol. 2011;6(11):1214–22. https://doi.org/10.1021/cb200105d.
Guiguemde WA, Shelat AA, Bouck D, Duffy S, Crowther GJ, Davis PH, et al. Chemical genetics of Plasmodium falciparum. Nature. 2010;465(7296):311–5. https://doi.org/10.1038/nature09099.
Ke H, Morrisey JM, Ganesan SM, Painter HJ, Mather MW, Vaidya AB. Variation among Plasmodium falciparum strains in their reliance on mitochondrial electron transport chain function. Eukaryot Cell. 2011;10(8):1053–61. https://doi.org/10.1128/EC.05049-11.
Biagini GA, Fisher N, Shone AE, Mubaraki MA, Srivastava A, Hill A, et al. Generation of quinolone antimalarials targeting the Plasmodium falciparum mitochondrial respiratory chain for the treatment and prophylaxis of malaria. Proc Natl Acad Sci U S A. 2012;109(21):8298–303. https://doi.org/10.1073/pnas.1205651109.
Boysen KE, Matuschewski K. Arrested oocyst maturation in Plasmodium parasites lacking type II NADH:ubiquinone dehydrogenase. J Biol Chem. 2011;286(37):32661–71. https://doi.org/10.1074/jbc.M111.269399.
•• Goodman CD, Siregar JE, Mollard V, Vega-Rodriguez J, Syafruddin D, Matsuoka H, et al. Parasites resistant to the antimalarial atovaquone fail to transmit by mosquitoes. Science. 2016;352(6283):349–53. https://doi.org/10.1126/science.aad9279. This study confirmed that there is a fitness cost associated with drug resistance. Atovaquone resistant parasites are not transmitted by mosquitoes, suggesting that atovaquone or other drugs that target pathways important for the parasite insect stage may be useful to help prevent the spread of resistance.
Mather MW, Darrouzet E, Valkova-Valchanova M, Cooley JW, McIntosh MT, Daldal F, et al. Uncovering the molecular mode of action of the antimalarial drug atovaquone using a bacterial system. J Biol Chem. 2005;280(29):27458–65. https://doi.org/10.1074/jbc.M502319200.
Fisher N, Abd Majid R, Antoine T, Al-Helal M, Warman AJ, Johnson DJ, et al. Cytochrome b mutation Y268S conferring atovaquone resistance phenotype in malaria parasite results in reduced parasite bc1 catalytic turnover and protein expression. J Biol Chem. 2012;287(13):9731–41. https://doi.org/10.1074/jbc.M111.324319.
Ke H, Lewis IA, Morrisey JM, McLean KJ, Ganesan SM, Painter HJ, et al. Genetic investigation of tricarboxylic acid metabolism during the Plasmodium falciparum life cycle. Cell Rep. 2015;11(1):164–74. https://doi.org/10.1016/j.celrep.2015.03.011.
Hino A, Hirai M, Tanaka TQ, Watanabe Y, Matsuoka H, Kita K. Critical roles of the mitochondrial complex II in oocyst formation of rodent malaria parasite Plasmodium berghei. J Biochem. 2012;152(3):259–68. https://doi.org/10.1093/jb/mvs058.
Tanaka TQ, Hirai M, Watanabe Y, Kita K. Toward understanding the role of mitochondrial complex II in the intraerythrocytic stages of Plasmodium falciparum: gene targeting of the Fp subunit. Parasitol Int. 2012;61(4):726–8. https://doi.org/10.1016/j.parint.2012.06.002.
Guler JL, Freeman DL, Ahyong V, Patrapuvich R, White J, Gujjar R, et al. Asexual populations of the human malaria parasite, Plasmodium falciparum, use a two-step genomic strategy to acquire accurate, beneficial DNA amplifications. PLoS Pathog. 2013;9(5):e1003375. https://doi.org/10.1371/journal.ppat.1003375.
Gonczarowska-Jorge H, Zahedi RP, Sickmann A. The proteome of baker’s yeast mitochondria. Mitochondrion. 2016 https://doi.org/10.1016/j.mito.2016.08.007.
Calvo SE, Clauser KR, Mootha VK. MitoCarta2.0: an updated inventory of mammalian mitochondrial proteins. Nucleic Acids Res. 2016;44(D1):D1251–7. https://doi.org/10.1093/nar/gkv1003.
Surolia N, Padmanaban G. De novo biosynthesis of heme offers a new chemotherapeutic target in the human malarial parasite. Biochem Biophys Res Commun. 1992;187(2):744–50.
Padmanaban G, Rangarajan PN. Heme metabolism of Plasmodium is a major antimalarial target. Biochem Biophys Res Commun. 2000;268(3):665–8. https://doi.org/10.1006/bbrc.1999.1892.
Padmanaban G, Nagaraj VA, Rangarajan PN. An alternative model for heme biosynthesis in the malarial parasite. Trends Biochem Sci. 2007;32(10):443–9. https://doi.org/10.1016/j.tibs.2007.09.005.
Padmanaban G, Rangarajan PN. Emerging targets for antimalarial drugs. Expert Opin Ther Targets. 2001;5(4):423–41. https://doi.org/10.1517/14728222.5.4.423.
van Dooren GG, Kennedy AT, McFadden GI. The use and abuse of heme in apicomplexan parasites. Antioxid Redox Signal. 2012;17(4):634–56. https://doi.org/10.1089/ars.2012.4539.
Ke H, Sigala PA, Miura K, Morrisey JM, Mather MW, Crowley JR, et al. The heme biosynthesis pathway is essential for Plasmodium falciparum development in mosquito stage but not in blood stages. J Biol Chem. 2014;289(50):34827–37. https://doi.org/10.1074/jbc.M114.615831.
Nagaraj VA, Sundaram B, Varadarajan NM, Subramani PA, Kalappa DM, Ghosh SK, et al. Malaria parasite-synthesized heme is essential in the mosquito and liver stages and complements host heme in the blood stages of infection. PLoS Pathog. 2013;9(8):e1003522. https://doi.org/10.1371/journal.ppat.1003522.
MacRae JI, Dixon MW, Dearnley MK, Chua HH, Chambers JM, Kenny S, et al. Mitochondrial metabolism of sexual and asexual blood stages of the malaria parasite Plasmodium falciparum. BMC Biol. 2013;11:67. https://doi.org/10.1186/1741-7007-11-67.
Cobbold SA, Vaughan AM, Lewis IA, Painter HJ, Camargo N, Perlman DH, et al. Kinetic flux profiling elucidates two independent acetyl-CoA biosynthetic pathways in Plasmodium falciparum. J Biol Chem. 2013;288(51):36338–50. https://doi.org/10.1074/jbc.M113.503557.
Storm J, Sethia S, Blackburn GJ, Chokkathukalam A, Watson DG, Breitling R, et al. Phosphoenolpyruvate carboxylase identified as a key enzyme in erythrocytic Plasmodium falciparum carbon metabolism. PLoS Pathog. 2014;10(1):e1003876. https://doi.org/10.1371/journal.ppat.1003876.
Foth BJ, Stimmler LM, Handman E, Crabb BS, Hodder AN, McFadden GI. The malaria parasite Plasmodium falciparum has only one pyruvate dehydrogenase complex, which is located in the apicoplast. Mol Microbiol. 2005;55(1):39–53. https://doi.org/10.1111/j.1365-2958.2004.04407.x.
Oppenheim RD, Creek DJ, Macrae JI, Modrzynska KK, Pino P, Limenitakis J, et al. BCKDH: the missing link in apicomplexan mitochondrial metabolism is required for full virulence of Toxoplasma gondii and Plasmodium berghei. PLoS Pathog. 2014;10(7):e1004263. https://doi.org/10.1371/journal.ppat.1004263.
Bulusu V, Jayaraman V, Balaram H. Metabolic fate of fumarate, a side product of the purine salvage pathway in the intraerythrocytic stages of Plasmodium falciparum. J Biol Chem. 2011;286(11):9236–45. https://doi.org/10.1074/jbc.M110.173328.
Bulusu V, Srinivasan B, Bopanna MP, Balaram H. Elucidation of the substrate specificity, kinetic and catalytic mechanism of adenylosuccinate lyase from Plasmodium falciparum. Biochim Biophys Acta. 2009;1794(4):642–54. https://doi.org/10.1016/j.bbapap.2008.11.021.
Woods SA, Schwartzbach SD, Guest JR. Two biochemically distinct classes of fumarase in Escherichia coli. Biochim Biophys Acta. 1988;954(1):14–26.
Flint DH, Emptage MH, Guest JR. Fumarase a from Escherichia coli: purification and characterization as an iron-sulfur cluster containing enzyme. Biochemistry. 1992;31(42):10331–7.
van Dooren GG, Stimmler LM, McFadden GI. Metabolic maps and functions of the Plasmodium mitochondrion. FEMS Microbiol Rev. 2006;30(4):596–630. https://doi.org/10.1111/j.1574-6976.2006.00027.x.
Prommana P, Uthaipibull C, Wongsombat C, Kamchonwongpaisan S, Yuthavong Y, Knuepfer E, et al. Inducible knockdown of Plasmodium gene expression using the glmS ribozyme. PLoS One. 2013;8(8):e73783. https://doi.org/10.1371/journal.pone.0073783.
Ganesan SM, Falla A, Goldfless SJ, Nasamu AS, Niles JC. Synthetic RNA-protein modules integrated with native translation mechanisms to control gene expression in malaria parasites. Nat Commun. 2016;7:10727. https://doi.org/10.1038/ncomms10727.
Preiser PR, Wilson RJ, Moore PW, McCready S, Hajibagheri MA, Blight KJ, et al. Recombination associated with replication of malarial mitochondrial DNA. EMBO J. 1996;15(3):684–93.
Ji YE, Mericle BL, Rehkopf DH, Anderson JD, Feagin JE. The Plasmodium falciparum 6 kb element is polycistronically transcribed. Mol Biochem Parasitol. 1996;81(2):211–23.
Suplick K, Morrisey J, Vaidya AB. Complex transcription from the extrachromosomal DNA encoding mitochondrial functions of Plasmodium yoelii. Mol Cell Biol. 1990;10(12):6381–8.
Ke H, Morrisey JM, Ganesan SM, Mather MW, Vaidya AB. Mitochondrial RNA polymerase is an essential enzyme in erythrocytic stages of Plasmodium falciparum. Mol Biochem Parasitol. 2012;185(1):48–51. https://doi.org/10.1016/j.molbiopara.2012.05.001.
Gupta A, Shah P, Haider A, Gupta K, Siddiqi MI, Ralph SA, et al. Reduced ribosomes of the apicoplast and mitochondrion of Plasmodium spp. and predicted interactions with antibiotics. Open Biol. 2014;4(5):140045. https://doi.org/10.1098/rsob.140045.
Greber BJ, Boehringer D, Leitner A, Bieri P, Voigts-Hoffmann F, Erzberger JP, et al. Architecture of the large subunit of the mammalian mitochondrial ribosome. Nature. 2014;505(7484):515–9. https://doi.org/10.1038/nature12890.
Kaushal PS, Sharma MR, Booth TM, Haque EM, Tung CS, Sanbonmatsu KY, et al. Cryo-EM structure of the small subunit of the mammalian mitochondrial ribosome. Proc Natl Acad Sci U S A. 2014;111(20):7284–9. https://doi.org/10.1073/pnas.1401657111.
Zikova A, Panigrahi AK, Dalley RA, Acestor N, Anupama A, Ogata Y, et al. Trypanosoma brucei mitochondrial ribosomes: affinity purification and component identification by mass spectrometry. Mol Cell Proteomics. 2008;7(7):1286–96. https://doi.org/10.1074/mcp.M700490-MCP200.
Feagin JE, Harrell MI, Lee JC, Coe KJ, Sands BH, Cannone JJ, et al. The fragmented mitochondrial ribosomal RNAs of Plasmodium falciparum. PLoS One. 2012;7(6):e38320. https://doi.org/10.1371/journal.pone.0038320.
Haider A, Allen SM, Jackson KE, Ralph SA, Habib S. Targeting and function of proteins mediating translation initiation in organelles of Plasmodium falciparum. Mol Microbiol. 2015;96(4):796–814. https://doi.org/10.1111/mmi.12972.
Gupta A, Mir SS, Saqib U, Biswas S, Vaishya S, Srivastava K, et al. The effect of fusidic acid on Plasmodium falciparum elongation factor G (EF-G). Mol Biochem Parasitol. 2013;192(1–2):39–48. https://doi.org/10.1016/j.molbiopara.2013.10.003.
Vaishya S, Kumar V, Gupta A, Siddiqi MI, Habib S. Polypeptide release factors and stop codon recognition in the apicoplast and mitochondrion of Plasmodium falciparum. Mol Microbiol. 2016;100(6):1080–95. https://doi.org/10.1111/mmi.13369.
Gupta A, Mir SS, Jackson KE, Lim EE, Shah P, Sinha A, et al. Recycling factors for ribosome disassembly in the apicoplast and mitochondrion of Plasmodium falciparum. Mol Microbiol. 2013;88(5):891–905. https://doi.org/10.1111/mmi.12230.
Habib S, Vaishya S, Gupta K. Translation in organelles of apicomplexan parasites. Trends Parasitol. 2016. doi:https://doi.org/10.1016/j.pt.2016.07.005.
Kessl JJ, Lange BB, Merbitz-Zahradnik T, Zwicker K, Hill P, Meunier B, et al. Molecular basis for atovaquone binding to the cytochrome bc1 complex. J Biol Chem. 2003;278(33):31312–8. https://doi.org/10.1074/jbc.M304042200.
Beinert H. Iron-sulfur proteins: ancient structures, still full of surprises. J Biol Inorg Chem. 2000;5(1):2–15.
Beinert H, Holm RH, Munck E. Iron-sulfur clusters: nature’s modular, multipurpose structures. Science. 1997;277(5326):653–9.
Tachezy J, Sanchez LB, Muller M. Mitochondrial type iron-sulfur cluster assembly in the amitochondriate eukaryotes Trichomonas vaginalis and Giardia intestinalis, as indicated by the phylogeny of IscS. Mol Biol Evol. 2001;18(10):1919–28.
• Karnkowska A, Vacek V, Zubacova Z, Treitli SC, Petrzelkova R, Eme L, et al. A eukaryote without a mitochondrial organelle. Curr Biol. 2016;26(10):1274–84. https://doi.org/10.1016/j.cub.2016.03.053. This study discovered the first eukaryote, Monocercomonoides sp , without a mitochondrion or a mitochondrion derivative organelle. The essential mitochondrial iron-sulfur cluster biosynthesis pathway is replaced by a cytosolic pathway encoded by bacterial genes acquired through lateral gene transfer.
Mather MW, Henry KW, Vaidya AB. Mitochondrial drug targets in apicomplexan parasites. Curr Drug Targets. 2007;8(1):49–60.
Stehling O, Wilbrecht C, Lill R. Mitochondrial iron-sulfur protein biogenesis and human disease. Biochimie. 2014;100:61–77. https://doi.org/10.1016/j.biochi.2014.01.010.
Gisselberg JE, Dellibovi-Ragheb TA, Matthews KA, Bosch G, Prigge ST. The suf iron-sulfur cluster synthesis pathway is required for apicoplast maintenance in malaria parasites. PLoS Pathog. 2013;9(9):e1003655. https://doi.org/10.1371/journal.ppat.1003655.
Nyakundi DO, Vuko LA, Bentley SJ, Hoppe H, Blatch GL, Boshoff A. Plasmodium falciparum Hep1 is required to prevent the self aggregation of PfHsp70-3. PLoS One. 2016;11(6):e0156446. https://doi.org/10.1371/journal.pone.0156446.
Bhaduri-McIntosh S, Vaidya AB. Molecular characterization of a Plasmodium falciparum gene encoding the mitochondrial phosphate carrier. Mol Biochem Parasitol. 1996;78(1–2):297–301.
Bhaduri-McIntosh S, Vaidya AB. Plasmodium falciparum: import of a phosphate carrier protein into heterologous mitochondria. Exp Parasitol. 1998;88(3):252–4. https://doi.org/10.1006/expr.1998.4242.
Hatin I, Jambou R, Ginsburg H, Jaureguiberry G. Single or multiple localization of ADP/ATP transporter in human malarial Plasmodium falciparum. Biochem Pharmacol. 1992;43(1):71–5.
Hatin I, Jaureguiberry G. Molecular characterisation of the ADP/ATP-transporter cDNA from the human malaria parasite Plasmodium falciparum. Eur J Biochem. 1995;228(1):86–91.
Jambou R, Hatin I, Jaureguiberry G. Evidence by in situ hybridization for stage-specific expression of the ATP/ADP translocator mRNA in Plasmodium falciparum. Exp Parasitol. 1995;80(3):568–71. https://doi.org/10.1006/expr.1995.1070.
Nozawa A, Fujimoto R, Matsuoka H, Tsuboi T, Tozawa Y. Cell-free synthesis, reconstitution, and characterization of a mitochondrial dicarboxylate-tricarboxylate carrier of Plasmodium falciparum. Biochem Biophys Res Commun. 2011;414(3):612–7. https://doi.org/10.1016/j.bbrc.2011.09.130.
Pino P, Aeby E, Foth BJ, Sheiner L, Soldati T, Schneider A, et al. Mitochondrial translation in absence of local tRNA aminoacylation and methionyl tRNA Met formylation in Apicomplexa. Mol Microbiol. 2010;76(3):706–18. https://doi.org/10.1111/j.1365-2958.2010.07128.x.
Sharma A, Sharma A. Plasmodium falciparum mitochondria import tRNAs along with an active phenylalanyl-tRNA synthetase. Biochem J. 2015;465(3):459–69. https://doi.org/10.1042/BJ20140998.
Tonhosolo R, D'Alexandri FL, Genta FA, Wunderlich G, Gozzo FC, Eberlin MN, et al. Identification, molecular cloning and functional characterization of an octaprenyl pyrophosphate synthase in intra-erythrocytic stages of Plasmodium falciparum. Biochem J. 2005;392(Pt 1):117–26. https://doi.org/10.1042/BJ20050441.
Tonhosolo R, D’Alexandri FL, de Rosso VV, Gazarini ML, Matsumura MY, Peres VJ, et al. Carotenoid biosynthesis in intraerythrocytic stages of Plasmodium falciparum. J Biol Chem. 2009;284(15):9974–85. https://doi.org/10.1074/jbc.M807464200.
Jenkins BJ, Daly TM, Morrisey JM, Mather MW, Vaidya AB, Bergman LW. Characterization of a plasmodium falciparum orthologue of the yeast ubiquinone-binding protein, Coq10p. PLoS One. 2016;11(3):e0152197. https://doi.org/10.1371/journal.pone.0152197.
•• Sidik SM, Huet D, Ganesan SM, Huynh MH, Wang T, Nasamu AS, et al. A genome-wide CRISPR screen in Toxoplasma identifies essential apicomplexan genes. Cell. 2016;166(6):1423–35 e12. https://doi.org/10.1016/j.cell.2016.08.019. This is the first genome-wide knockout screening carried out by a CRISPR-Cas9 system in an apicomplexan parasite. The feasibility of CRISPR-Cas9 knockout in Toxoplasma allowed the discovery of essential genes that were not previously characterized, termed “indispensable conserved apicomplexan proteins” (ICAP). Studies on these ICAP genes may identify novel essential pathways of apicomplexan parasites.
Acknowledgements
We thank our colleagues at the Center for Parasitology at Drexel University College of Medicine for comments and advice.
Funding
Writing this review was supported by a NIH grant to Dr. Akhil B. Vaidya (AI028398) at the Center for Parasitology at Drexel University College of Medicine.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Dr. Hangjun Ke and Dr. Michael W. Mather are supported by an NIH grant (AI028398) to Dr. Akhil B. Vaidya.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Parasitology
Rights and permissions
About this article
Cite this article
Ke, H., Mather, M.W. +Targeting Mitochondrial Functions as Antimalarial Regime, What Is Next?. Curr Clin Micro Rpt 4, 175–191 (2017). https://doi.org/10.1007/s40588-017-0075-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40588-017-0075-5