Abstract
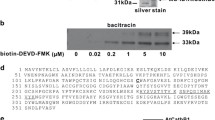
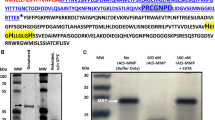
The proteolytic machinery of plant organelles is largely unknown, although indications so far point to several proteases of bacterial origin. In this study an Arabidopsis thaliana cDNA was isolated that encodes a homologue of bacterial ClpX, a molecular chaperone and regulatory subunit of the ATP-dependent, serine-type Clp protease. Computer analysis of the predicted plant ClpX revealed a putative mitochondrial transit peptide at the N-terminus, as well as overall sequence similarity to other eukaryotic ClpX homologues. Specific polyclonal antibodies were made to the Arabidopsis ClpX protein and used to confirm its localization in plant mitochondria. In addition to ClpX, a ClpP protein located in mitochondria was also identified from the numerous ClpP isomers in Arabidopsis. Localization of this nuclear-encoded protein, termed ClpP2, was determined first by its close sequence similarity to mitochondrial ClpP human, and later experimentally using ClpP2-specific antibodies with isolated plant organellar fractions. In Arabidopsis, transcripts for both clpX and clpP2 genes were detected in various tissues and under different growth conditions, with no significant variation in mRNA level (i.e. 2-fold) for each gene between samples. Using β-casein as a substrate, plant mitochondria were found to possess an ATP-stimulated, serine-type proteolytic activity that could be strongly inhibited by antibodies specific for ClpX or ClpP2, suggesting an active ClpXP protease. The recent discovery of homologous mitochondrial ClpX and ClpP proteins in mammals suggests that this type of protease may be common to multicellular eukaryotes.
Similar content being viewed by others
References
Adam, Z., Adamska, I., Nakabayashi, K., Ostersetzer, O., Haussuhl,K., Manuell, A., Zheng, B., Vallon, O., Rodermel, S.R., Shinozaki, K. and Clarke A.K. 2001. Chloroplast and mitochondrial proteases in Arabidopsis thaliana: a proposed nomenclature. Plant Physiol. (in press).
Beynon, R.J. and Bond, J.S. 1989. Proteolytic enzymes: a practical approach. IRL Press.
Clarke, A.K. 1999. ATP-dependent Clp proteases in photosynthetic organisms - A cut above the rest! Ann. Bot. 83: 593-599.
Clarke, A.K., Gustafsson, P. and Lidholm, J.A. 1994. Identification and expression of the chloroplast clpP gene in the conifer Pinus contorta. Plant Mol. Biol. 26: 851-862
Corydon, T.J., Bross, P., Holst, H.U., Neve, S., Kristiansen, K., Gregersen, N. and Bolund, L. 1998. A human homologue of Escherichia coli ClpP caseinolytic protease: recombinant expression, intracellular processing and subcellular localization. Biochem. J. 331: 309-316.
de Sagarra, M.R., Mayo, I., Marco, S., Rodriguez-Vilarino, S., Oliva, J., Carrascosa, J.L. and Casta, J.G. 1999. Mitochondrial localization and oligomeric structure of HClpP, the human homologue of E. coli clpP. J. Mol. Biol. 29: 819-825.
Desimone, M., Weiss-Wichert, W., Wagner, E., Altenfeld, U. and Johanningmeier, U. 1997. Immunochemical studies on the Clp-protease in chloroplasts: evidence for the formation of a ClpC/P complex. Bot. Acta 11: 234-239.
Gottesman, S. 1996. Proteases and their targets in Escherichia coli. Annu. Rev. Genet. 30: 465-506.
Gottesman, S., Clark, W.P., de Crecy-Lagard, V. and Maurizi, M.R. 1993. ClpX, an alternative subunit for the ATP-dependent Clp protease of Escherichia coli: sequence and in vivo activities. J. Biol. Chem. 268: 22618-22626.
Gottesman, S., Maurizi, M.R. and Wickner, S. 1997. Regulatory subunits of energy-dependent proteases. Cell 91:435-438.
Gottesman, S., Squires, C., Pichersky, E., Carrington, M., Hobbs, M., Mattick, J.S., Dalrymple, B., Kuramitsu, H., Shiroza, T., Foster, T., Clark, W.P., Ross, B., Squires, C.L., Maurizi, M.R. 1990. Conservation of the regulatory subunit for the Clp ATP-dependent protease in prokaryotes and eukaryotes. Proc. Natl. Acad. Sci. USA 87: 3513-3517.
Gottesman, S., Wickner, S. and Maurizi, M.R. 1997. Protein quality control: triage by chaperones and proteases. Genes Dev. 11: 815-823.
Gray, J.C., Hird, S.M. and Dyer, T.A. 1990. Nucleotide sequence of a wheat chloroplast gene encoding the proteolytic subunit of an ATP-dependent protease. Plant Mol. Biol. 15: 947-950.
Halperin, T. and Adam, Z. 1996. Degradation of mistargeted OEE33 in the chloroplast stroma. Plant Mol. Biol. 30: 925-933.
Hamasur, B., Birgersson, U., Eriksson, A.C. and Glaser, E. 1990. Large-scale purification procedure of spinach leaf mitochondria- isolation and immunological studies of the F1-ATPase. Physiol. Plant. 78: 367-373.
Harlow, E. and Lane, D. 1988. Antibodies: A Laboratory Manual. Cold Spring Harbor Laboratory, Plainview, NY.
Horwich, A.L., Weber-Ban, E.U. and Finley, D. 1999. Chaperone rings in protein folding and degradation. Proc. Natl. Acad. Sci. USA 96: 11033-11040.
Jenal, U. and Fuchs, T. 1998. An essential protease involved in bacterial cell-cycle control. EMBO J. 17: 5658-5669.
Kessel, M., Maurizi, M.R., Kim, B., Kocsis, E., Trus, B.L., Singh, S.K. and Steven, A.C. 1995. Homology in structural organization between E. coli ClpAP protease and the eukaryotic 26S proteasome. J. Mol. Biol. 250: 587-594.
Kiyosue, T., Yamaguchi-Shinozaki, K. and Shinozaki, K. 1993. Characterization of cDNA for a dehydration-inducible gene that encodes a CLP A, B-like protein in Arabidopsis thaliana L. Biochem. Biophys. Res. Commun. 196: 1214-1220.
Levchenko, I., Luo, L. and Baker, T.A. 1995. Disassembly of the mu transposase tetramer by the ClpX chaperone. Gene Develop. 9: 2399-2408.
Levy, M. and Adam, Z. 1995. Mutations in the processing site of the precursor of ribulose-1,5-bisphosphate carboxylase oxygenase small subunit: effects on import, processing, assembly and stability. Plant Mol. Biol. 29: 53-61.
Maurizi, M.R., Clark, W.P., Kim, S.H. and Gottesman, S. 1990. ClpP represents a unique family of serine proteases. J. Biol. Chem. 265: 12546-12552.
Moore, T. and Keegstra, K. 1993. Characterization of a cDNA clone encoding a chloroplast-targeted Clp homologue. Plant Mol. Biol. 21: 525-537.
Nielsen, E., Akita, M., Davilaaponte, J. and Keegstra, K. 1997. Stable association of chloroplastic precursors with protein translocation complexes that contain proteins from both envelope membranes and a stromal hsp 100 molecular chaperone. EMBO J. 16: 935-946.
Ostersetzer, O. and Adam, Z. 1996. Effects of light and temperature on expression of ClpC, the regulatory subunit of chloroplastic Clp protease, in pea seedlings. Plant Mol. Biol. 31: 673-676.
Ostersetzer, O., Tabak, S., Yarden, O., Shapira, R. and Adam, Z. 1996. Immunological detection of proteins similar to bacterial proteases in higher plant chloroplasts. Eur. J. Biochem. 236: 932-936.
Porankiewicz, J., Wang, J. and Clarke, A.K. 1999. New insights into the ATP-dependent Clp protease: Escherichia coli and beyond. Mol. Microbiol. 32: 449-458.
Santagata, S., Bhattacharyya, D., Wang, F.H., Singha, N., Hodtsev, A. and Spanopoulou, E. 1999. Molecular cloning and characterization of a mouse homolog of bacterial ClpX, a novel mammalian class II member of the Hsp100/Clp chaperone family. J. Biol. Chem. 274: 16311-16319.
Sarria, R., Lyznik, A., Vallejos, C.E. and Mackenzie, S.A. 1998. A cytoplasmic male sterility-associated mitochondrial peptide in common bean is post-translationally regulated. Plant Cell 10: 1217-1228.
Shanklin, J., Dewitt, N.D. and Flanagan, J.M. 1995. The stroma of higher plant plastids contain ClpP and ClpC, functional homologs of Escherichia coli ClpP and ClpA: an archetypal two-component ATP-dependent protease. Plant Cell 7: 1713-1722.
Sokolenko, A., Lerbs-Mache, S., Altschmied, L. and Herrmann, R.G. 1998. Clp protease complexes and their diversity in chloroplasts. Planta 207: 286-295.
van Dyck, L., Dembowski, M., Neupert, W. and Langer, T. 1998. Mcx1p, a ClpX homologue in mitochondria of Saccharomyces cerevisiae. FEBS Lett 438: 250-254.
Wang, J., Hartling, J.A. and Flanagan, J.M. 1997. The structure of ClpP at 2.3 Å resolution suggests a model for ATP-dependent proteolysis. Cell 91: 447-456.
Weaver, L.M., Froehlich, J.E. and Amasino, R.M. 1999. Chloroplast-targeted ERD1 protein declines but its mRNA increases during senescence in Arabidopsis. Plant Physiol. 119: 1209-1216.
Wojtkowiak, D., Georgopulos, C. and Zylicz, M. 1993. ClpX, a new specificity component of the ATP-dependent Escherichia coli Clp protease, is potentially involved in λ DNA replication. J. Biol. Chem. 268: 22609-22617.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Halperin, T., Zheng, B., Itzhaki, H. et al. Plant mitochondria contain proteolytic and regulatory subunits of the ATP-dependent Clp protease. Plant Mol Biol 45, 461–468 (2001). https://doi.org/10.1023/A:1010677220323
Issue Date:
DOI: https://doi.org/10.1023/A:1010677220323