Abstract
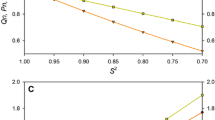
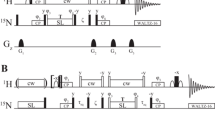
Although an accurate description of global tumbling of a protein is essential for correct analysis of internal motions, proper distinction between the effects of anisotropic rotational diffusion and conformational exchange has remained a challenge. We present a novel two-part filtering procedure designed specifically to distinguish between the effects of anisotropy and conformational exchange. The efficacy of this method is assessed using synthetic data sets. The method is then applied to two proteins of dramatically different size and shape, OspA and SH3. The large size and extreme anisotropy of OspA provide a challenging case, where conformational exchange is a small perturbation of the effects of anisotropy on transverse relaxation rates. Conversely, in the chicken c-Src SH3 domain, with its small size and nearly spherical shape, anisotropy is a small perturbation of the effects of conformational exchange on transverse relaxation rates. Accurate extraction of the global tumbling parameters for each protein allows optimal characterization of conformational exchange processes, as well as ps–ns time scale motions.
Similar content being viewed by others
References
Akke, M., Bruschweiler, R. and Palmer, A.G. (1993) J. Am. Chem. Soc., 115, 9832-9833.
Akke, M. and Palmer, A.G. (1996) J. Am. Chem. Soc., 118, 911-912.
Andrec, M., Montelione, G.T. and Levy, R.M. (1999) J. Magn. Reson., 139, 408-421.
Bevington, P.R. and Robinson, D.K. (1992) Data Reduction and Error Analysis for the Physical Sciences, McGraw-Hill, Boston, MA.
Bu, Z.M., Koide, S. and Engelman, D.M. (1998) Protein Sci., 7, 2681-2683.
Cavanagh, J., Fairbrother, W.J., Palmer, A.G. and Skelton, N.J. (1996) Protein NMR Spectroscopy: Principles and Practice, Academic Press, San Diego, CA.
Clore, G.M., Szabo, A., Bax, A., Kay, L.E., Driscoll, P.C. and Gronenborn, A.M. (1990) J. Am. Chem. Soc., 112, 4989-4991.
Cordier, F., Wang, C., Grzesiek, S. and Nicholson, L.K. (2000) J. Mol. Biol., 304, 497-505.
de Alba, E., Baber, J.L. and Tjandra, N. (1999) J. Am. Chem. Soc., 121, 4282-4283.
Farrar, T.C. and Becker, E.D. (1971) Pulse and Fourier Transform NMR: Introduction to Theory and Methods, Academic Press, New York, NY.
Farrow, N.A., Muhandiram, R., Singer, A.U., Pascal, S.M., Kay, C.M., Gish, G., Shoelson, S.E., Pawson, T., Forman-Kay, J.D. and Kay, L.E. (1994) Biochemistry, 33, 5984-6003.
Feher, V.A. and Cavanagh, J. (1999) Nature, 400, 289-293.
Forman-Kay, J.D. (1999) Nat. Struct. Biol., 6, 1086-1087.
Fushman, D., Ghose, R. and Cowburn, D. (2000) J. Am. Chem. Soc., 122, 10640-10649.
Grzesiek, S. and Bax, A. (1993) J. Am. Chem. Soc., 115, 12593-12594.
Ishima, R., Freedberg, D.I., Wang, Y.X., Louis, J.M. and Torchia, D.A. (1999) Struct. Fold. Des., 7, 1047-1055.
Jia, X., Lee, L.K., Light, J., Palmer, A.G. and Assa-Munt, N. (1999) J. Mol. Biol., 292, 1083-1093.
Kay, L.E., Torchia, D.A. and Bax, A. (1989) Biochemistry, 28, 8972-8979.
Kroenke, C.D., Loria, J.P., Lee, L.K., Rance, M. and Palmer, A.G. (1998) J. Am. Chem. Soc., 120, 7905-7915.
Lee, A.L. and Wand, A.J. (1999) J. Biomol. NMR, 13, 101-112.
Lee, L.K., Rance, M., Chazin, W.J. and Palmer, A.G. (1997) J. Biomol. NMR, 9, 287-298.
Li, H., Dunn, J.J., Luft, B.J. and Lawson, C.L. (1997) Proc. Natl. Acad. Sci. USA, 94, 3584-3589.
Lipari, G. and Szabo, A. (1982) J. Am. Chem. Soc., 104, 4546-4559.
Luginbuhl, P., Pervushin, K.V., Iwai, H. and Wüthrich, K. (1997) Biochemistry, 36, 7305-7312.
Mandel, A.M., Akke, M. and Palmer, A.G. (1995) J. Mol. Biol., 246, 144-163.
Nicholson, L.K., Kay, L.E., Baldisseri, D.M., Arango, J., Young, P.E., Bax, A. and Torchia, D.A. (1992) Biochemistry, 31, 5253-5263.
Nicholson, L.K., Yamazaki, T., Torchia, D.A., Grzesiek, S., Bax, A., Stahl, S.J., Kaufman, J.D., Wingfield, P.T., Lam, P.Y.S., Jadhav, P.K., Hodge, C.N., Domaille, P.J. and Chang, C.H. (1995) Nat. Struct. Biol., 2, 274-280.
Palmer, A.G., Wright, P.E. and Rance, M. (1991) Chem. Phys. Lett., 185, 41-46.
Pham, T.N., Koide, A. and Koide, S. (1998) Nat. Struct. Biol., 5, 115-119.
Pham, T.N. and Koide, S. (1998) J. Biomol. NMR, 11, 407-414.
Phan, I.Q.H., Boyd, J. and Campbell, I.D. (1996) J. Biomol. NMR, 8, 369-378.
Press, W.H., Teukolsky, S.A., Vetterling, W.T. and Flannery, B.P. (1996) Numerical Recipes in C: The Art of Scientific Computing, Press Syndicate of the University of Cambridge, New York, NY.
Raiford, D.S., Fisk, C.L. and Becker, E.D. (1979) Anal. Chem., 51, 2050-2051.
Schurr, J.M., Babcock, H.P. and Fujimoto, B.S. (1994) J. Magn. Reson., B105, 211-224.
Skrynnikov, N.R., Goto, N.K., Yang, D.W., Choy, W.Y., Tolman, J.R., Mueller, G.A. and Kay, L.E. (2000) J. Mol. Biol., 295, 1265-1273.
Taylor, J.R. (1982) An Introduction to Error Analysis: The Study of Uncertainties in Physical Measurements, Oxford University Press, Mill Valley, CA.
Tjandra, N., Feller, S.E., Pastor, R.W. and Bax, A. (1995) J. Am. Chem. Soc., 117, 12562-12566.
Tjandra, N., Wingfield, P., Stahl, S. and Bax, A. (1996) J. Biomol. NMR, 8, 273-284.
Woessner, D.E. (1962) J. Chem. Phys., 37, 647-654.
Xu, W., Doshi, A., Lei, M., Eck, M.J. and Harrison, S.C. (1999) Mol. Cell, 3, 629-638.
Yamazaki, T., Muhandiram, R. and Kay, L.E. (1994) J. Am. Chem. Soc., 116, 8266-8278.
Yang, D.W., Mok, Y.K., Forman-Kay, J.D., Farrow, N.A. and Kay, L.E. (1997) J. Mol. Biol., 272, 790-804.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Pawley, N.H., Wang, C., Koide, S. et al. An improved method for distinguishing between anisotropic tumbling and chemical exchange in analysis of 15N relaxation parameters. J Biomol NMR 20, 149–165 (2001). https://doi.org/10.1023/A:1011249816560
Issue Date:
DOI: https://doi.org/10.1023/A:1011249816560