Abstract
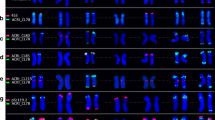
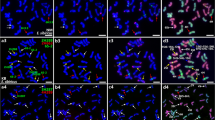
The ability to detect small low- or single-copy DNA sequences by fluorescence in-situ hybridization (FISH) is an important step towards physical mapping of plant genomes. In this study, the FISH technique was used to physically map the Glu-1 loci controlling high-molecular weight (HMW) glutenin in common wheat (Triticum aestivum cv. 'Chinese Spring') and tritordeum (am amphiploid between T. turgidum cv. durum and Hordeum chilense). The probe used was the single-copy Glu-D1-1d gene coding the 1Dx5 HMW glutenin subunit. Three loci were mapped on chromosomes of wheat homoeologous group 1 (arm 1AL, 1BL and 1DL). The Glu-1 loci were mapped (fraction of the distance from the centromere) at positions 0.76 ± 0.01, 0.69 ± 0.01 and 0.76 ± 0.01, on arms 1AL, 1BL and 1DL, respectively. The Glu-1 loci were also mapped on chromosomes of homoeologous group 1 of tritordeum at positions 0.75 ± 0.01, 0.70 ± 0.01 and 0.60 ± 0.01, on arms 1AL, 1BL and 1HchL, respectively. Chromosomes with positive signals were identified by reprobing chromosome preparations using both the GAA-satellite and pAs1 sequences simultaneously. The application of the FISH technique to study homoeology among different genomes is discussed.
Similar content being viewed by others
References
Alvarez JB, Martin A, Martin LM (2001) Variation in the high-molecular-weight glutenin subunits coded at the Glu-H ch1 locus in Hordeum chilense. Theor Appl Genet 102: 134–137.
Anderson OD, Greene FC, Yip RE, Halford NG, Shewry PR, Malpica-Romero JM (1989) Nucleotide sequences of the two high-molecular-weight glutenin genes from the D-genome of a hexaploid bread wheat, Triticum aestivum L Cheyenne. Nucleic Acids Res 17: 461–462.
Baum M, Appels R (1991) The cytogenetic and molecular architecture of chromosome 1R – one of the most widely utilized sources of alien chromatin in wheat varieties. Chromosoma 101: 1–10.
Cabrera A, Friebe B, Jiang J, Gill BS (1995) Characterization of Hordeum chilense chromosomes by C-banding and in situ hybridization using highly repeated DNA probes. Genome 38: 435–442.
Curtis CA, Lukaszewski AJ (1991) Genetic linkage between C-bands and storage protein genes in chromosome 1B of tetraploid wheat. Theor Appl Genet 81: 245–252.
Fransz PF, Stam M, Montijn B et al. (1996) Detection of single-copy genes and chromosome rearrangements in Petunia hybrida by fluorescence in situ hybridization. Plant J 9: 767–774.
Gill KS, Gill BS, Endo TR (1993) A chromosome region-specific mapping strategy reveals gene-rich telomeric ends in wheat. Chromosoma 102: 374–381.
Gustafson JP, Dillé JE (1992) Chromosome location of Oryza sativa recombination linkage groups. Proc Natl Acad Sci USA 89: 8646–8650.
Gustafson JP, Butler E, McIntyre CL (1990) Physical mapping of a low copy DNA sequence in rye (Secale cereale). Proc Natl Acad Sci USA 87: 1899–1902.
Halford NG, Forde J, Shewry PR, Kreis M (1989) Functional analysis of the upstreamregions of a silent and an expressed member of a family of wheat seed protein genes in transgenic tobacco. Plant Sci 62: 207–216.
Jiang J, Hulbert SH, Gill BS, Ward DC (1996) Interphase fluorescence in situ hybridization mapping: a physical mapping strategy for plant species with large complex genomes. Mol Gen Genet 252: 497–502.
Lehfer H, Busch W, Martin R, Herrmann RG (1993) Localization of the B-hordein locus on barley chromosomes using fluorescence in situ hybridization. Chromosoma 102: 428–432.
Leitch IJ, Heslop-Harrison JS (1993) Physical mapping of four sites of 5S rDNA sequences and one site of the alpha-amylase–2 gene in barley (Hordeum vulgare L.). Genome 36: 517–523.
Kota RS, Gill KS, Gill BS, Endo TR (1993) A cytogenetically based map of chromosome 1B in common wheat. Genome 36: 548–554.
Mukai Y, Nakahara Y, Yamamoto M (1993) Simultaneous discrimination of the three genomes in hexaploid wheat by multicolor fluorescence in situ hybridization using total and highly repeated DNA probes. Genome 36: 489–494.
Payne PI, Holt LM, Jackson EA, Law CN (1984) Wheat storage proteins: their genetics and their potential for manipulation by plant breeding. Phil Trans R Soc Lond B 304: 359–371.
Payne PI, Holt LM, Reader SM, Miller TE (1987) Chromosomal location of genes coding for endosperm proteins of Hordeum chilense, determined by two-dimensional electrophoresis of wheat–H. chilense chromosome addition lines. Biochem Genet 25: 53–65.
Pedersen C, Langridge P (1997) Identification of the entire chromosome complement of bread wheat by two-colour FISH. Genome 40: 589–593.
Pedersen C, Giese H, Linde-Laursen I (1995) Towards an integration of the physical and the genetic chromosome maps of barley by in situ hybridization. Hereditas 123: 77–88.
Pedersen C, Rasmussen SK, Linde-Laursen I (1996) Genome and chromosome identification in cultivated barley and related species of the Triticeae (Poaceae) by in situ hybridisation with the GAA-satellite sequence.Genome 39: 93–104.
Peterson DG, Lapitan NL, Stack SM (1999) Localization of single-and low-copy sequences on tomato synaptonemal complex spreads using fluorescence in situ hybridization (FISH). Genetics 152: 427–439.
Rayburn AL, Gill BS (1986) Isolation of a D-genome specific repeated DNA sequence from Aegilops squarrosa. Plant MolBiolRep 4: 102–109.
Salvo-Garrido H, Travella S, Schwarzacher T, Harwood WA, Snape JW (2001) An efficient method for the physical mapping of transgenes in barley using in situ hybridization. Genome 44: 104–110.
Simpson PR, Newman MA, Davies DR (1988) Detection of legumin gene sequences in pea by in situ hybridization. Chromosoma 96: 454–458.
Snape JW, Flavell RB, ÓDell M, Hughes WG, Payne PI (1985) Intrachromosomal mapping of the nucleolar organizer region relative to three marker loci on chromosome 1B of wheat (Triticum aestivum). Theor Appl Genet 69: 263–270.
Werner JE, Endo TR, Gill BS (1992) Toward a cytogenetically based physical map of the wheat genome. Proc Natl Acad Sci USA 89: 11307–11311.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Cabrera, A., Martín, A. & Barro, F. In-Situ Comparative Mapping (ISCM) of Glu-1 Loci in Triticum and Hordeum. Chromosome Res 10, 49–54 (2002). https://doi.org/10.1023/A:1014270227360
Issue Date:
DOI: https://doi.org/10.1023/A:1014270227360