Abstract
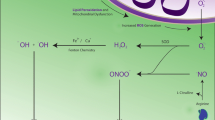
Central to oxidative damage in Alzheimer disease is the production of metal-catalyzed hydroxyl radicals that damage every category of macromolecule. Studies on redox-competent copper and iron indicate that redox activity in Alzheimer disease resides exclusively within the cytosol of vulnerable neurons and that chelation with deferoxamine or DTPA removes this activity. We have also found that while proteins that accumulate in Alzheimer disease such as tau, amyloid beta, and apolipoprotein E possess metal-binding sites, metal-associated cellular redox activity is more dependent on metal-nucleic acid binding. Consistent with this finding is the large amount of cytoplasmic RNA in pyramidal neurons. Still, the source of metal-catalyzed redox activity is controversial. Heme oxygenase-1, an enzyme that catalyzes the conversion of heme to iron and biliverdin, is increased in Alzheimer disease suggesting increased heme turnover as a source of redox-active iron. Additionally, the role of mitochondria as a potential source of redox-active metals and oxygen radical production is assuming more prominence. In recent studies, we have found that while mitochondrial DNA and cytochrome C oxidase activity are increased in Alzheimer disease, the number of mitochondria is decreased, indicating accelerated mitochondria turnover. This finding, as well as preliminary studies demonstrating a reduction in microtubule density in neurons in Alzheimer disease suggests mitochondrial dysfunction as a potentially inseparable component of the initiation and progression of Alzheimer disease.
Similar content being viewed by others
References
Aronov S, Aranda G, Behar L, Ginzburg I. 2001 Axonal tau mRNA localization coincides with tau protein in living neuronal cells and depends on axonal targeting signal. J Neurosci 21, 6577-6587.
Cuajungco MP, Goldstein LE, Nunomura A et al. 2000 Evidence that the β-amyloid plaques of Alzheimer's disease represent the redox-silencing and entombment of Aβ by zinc. J Biol Chem 275, 19439-19442.
Beckman JS, Ye YZ, Anderson PG et al. 1994 Extensive nitration of protein tyrosines in human atherosclerosis detected by immunohistochemistry. Biol Chem Hoppe-Seyler 375, 81-88.
Hirai K, Aliev G, Nunomura A et al. 2001. Mitochondrial abnormalities in Alzheimer's disease. J Neurosci 21, 3017-3023.
Keyse SM, Tyrrell RM. 1989 Heme oxygenase is the major 32-kDa stress protein induced in human skin fibroblasts by UVA radiation, hydrogen peroxide, and sodium arsenite. Proc Natl Acad Sci USA 86, 99-103.
Ledesma MD, Bonay P, Colaco C, Avila J. 1994 Analysis of microtubule-associated protein tau glycation in paired helical filaments. J Biol Chem 269, 21614-21619.
Mattson MP, Carney JW, Butterfield DA. 1995 A tombstone in Alzheimer's? Nature 373, 481.
Miyata M, Smith JD. 1996 Apolipoprotein E allele-specific antioxidant activity and effects on cytotoxicity by oxidative insults and beta-amyloid peptides. Nature Gen 14, 55-61.
Nunomura A, Perry G, Pappolla MA et al. 1999 RNA oxidation is a prominent feature of vulnerable neurons in Alzheimer's disease. J Neurosci 19, 1959-1964.
Perry G, Castellani RJ, Hirai K, Smith MA. 1998. Reactive oxygen species mediate cellular damage in Alzheimer disease. J Alzheimer Dis 1, 45-55.
Praprotnik D, Smith MA, Richey PL, Vinters HV, Perry G. 1996. Filament heterogeneity within the dystrophic neurites of senile plaques suggests blockage of fast axonal transport in Alzheimer's disease. Acta Neuropathol 91, 226-235.
Premkumar DRD, Smith MA, Richey PL et al. 1995 Induction of heme oxygenase-1 mRNA and protein in neocortex and cerebral vessels in Alzheimer's disease. J Neurochem 65, 1399-1402.
Sampson JB, Ye Y, Rosen H, Beckman JS. 1998 Myeloperoxidase and horseradish peroxidase catalyze tyrosine nitration in proteins from nitrite and hydrogen peroxide. Arch Biochem Biophys 356, 207-213.
Sayre LM, Zelasko DA, Harris PLR, Perry G, Salomon RG, Smith MA. 1997 4-Hydroxynonenal-derived advanced lipid peroxidation end products are increased in Alzheimer's disease. J Neurochem 68, 2092-2097.
Sayre LM, Perry G, Smith MA. 1999 In situ methods for detection and localization of markers of oxidative stress: application in neurodegenerative disorders. In: Wetzel R, ed. Methods of Enzymology, Vol. 309. San Diego: Academic Press; 133-152.
Sayre LM, Perry G, Harris PLR, Liu Y, Schubert KA, Smith MA. 2000 In situ oxidative catalysis by neurofibrillary tangles and senile plaques in Alzheimer's disease: a central role for bound transition metals. J Neurochem 74, 270-279.
Schipper HM, Cisse S, Stopa EG. 1995 Expression of heme oxygenase-1 in the senescent and Alzheimer-diseased brain. Ann Neurol 37, 758-768.
Smith MA, Kutty RK, Richey PL et al. 1994 Heme oxygenase-1 is associated with the neurofibrillary pathology of Alzheimer's disease. Am J Pathol 145, 42-47.
Smith MA, Sayre LM, Vitek MP, Monnier VM, Perry G. 1995 Early Ageing and Alzheimer's. Nature 374, 316.
Smith MA, Perry G, Richey PL et al. 1996 Oxidative damage in Alzheimer's. Nature 382, 120-121.
Smith MA, Harris PLR, Sayre LM, Beckman JS, Perry G. 1997 Widespread peroxynitrite-mediated damage in Alzheimer's disease. J Neurosci 17, 2653-2657.
Stieber A, Mourelatos Z, Gonatas NK. 1996 In Alzheimer's disease the Golgi apparatus of a population of neurons without neurofibrillary tangles is fragmented and atrophic. Am J Pathol 148, 415-426.
Suzuki K, Terry RD. 1967 Fine structural localization of acid phosphatase in senile plaques in Alzheimer's presenile dementia. Acta Neuropathol 8, 276-284.
Terry RD. 1996 The pathogenesis of Alzheimer disease: an alternative to the amyloid hypothesis. J Neuropathol Exp Neurol 55, 1023-1025.
Terry RD. 2000 Cell death or synaptic loss in Alzheimer disease. J Neuropathol Exp Neurol 59, 1118-1119.
Terry RD, Katzman R. 2001 Life span and synapses: will there be a primary senile dementia? Neurobiol Aging 22, 347-348.
Wataya T, Nunomura A, Smith MA et al. 2002 High molecular weight neurofilament proteins are physiological substrates of adduction by the lipid peroxidation product hydroxynonenal. J Biol Chem 277: 4644-4648.
Wong-Riley M, Antuono P, Ho K-V et al. 1997 Cytochrome oxidase in Alzheimer's disease: biochemical, histochemical, and immunohistochemical analyses of the visual and other systems. Vision Res 37, 3593-3608.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Perry, G., Taddeo, M.A., Petersen, R.B. et al. Adventiously-bound redox active iron and copper are at the center of oxidative damage in Alzheimer disease. Biometals 16, 77–81 (2003). https://doi.org/10.1023/A:1020731021276
Issue Date:
DOI: https://doi.org/10.1023/A:1020731021276