Abstract
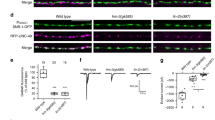
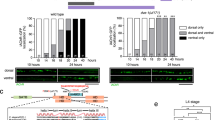
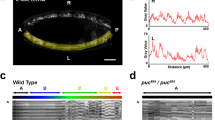
In the nematode Caenorhabditis elegans six GABAergic motor neurons, known as DDs1,2, remodel their patterns of synaptic connectivity during larval development3. DD remodelling involves a complete reversal of the direction of information flow within nerve processes without marked changes in process morphology. We used a marker localized in vivo to DD presynaptic zones to analyse how the timing of DD remodelling is controlled. In wild-type animals, DDs remodel their synaptic outputs within a 3–5-hour period at the end of the first larval stage. We show that the heterochronic gene lin-14, which controls the timing of stage-specific cell lineages4,5, regulates the timing of DD synaptic output remodelling. In lin-14 loss-of-function mutants, DDs remodel precociously. The degree of precocious remodelling is correlated with the level of lin-14 activity. Expression of lin-14(+) in the DDs of lin-14-null mutants rescues the precocious remodelling, indicating that lin-14 can act cell-autonomously. Consistent with this hypothesis, LIN-14 protein levels decrease in the DDs before remodelling. Our observations reveal a role of heterochronic genes in non-dividing cells, and provide an example of cell-autonomous respecification of neuronal connectivity.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
McIntire, S. L., Jorgensen, E., Kaplan, J. & Horvitz, H. R. The GABAergic nervous system of Caenorhabditis elegans. Nature 364, 337–341 (1993).
White, J. G., Southgate, E., Thomson, J. N. & Brenner, S. The structure of the nervous system of the nematode Caenorhabditis elegans. Phil. Trans. R. Soc. Lond. B 314, 1–340 (1986).
White, J. G., Albertson, D. G. & Anness, M. A. R. Connectivity changes in a class of motorneurons during the development of a nematode. Nature 271, 764–766 (1978).
Ambros, V. & Horvitz, H. R. Heterochronic mutants of the nematode Caenorhabditis elegans. Science 226, 409–416 (1984).
Ambros, V. & Horvitz, H. R. The lin-14 locus of Caenorhabditis elegans controls the time of expression of specific postembryonic developmental events. Genes Dev. 1, 398–414 (1987).
Harris, W. A. Neurometamorphosis. J. Neurobiol. 21, 953–957 (1990).
Truman, J. Metamorphosis of the central nervous system of Drosophila. J. Neurobiol. 21, 1072–1084 (1990).
Levine, R. B., Morton, D. B. & Restifo, L. L. Remodeling of the insect nervous system. Curr. Opin. Neurobiol. 5, 28–35 (1995).
Alley, K. E. Retrofitting larval neuromuscular circuits in the metamorphosing frog. J. Neurobiol. 21, 1092–1107 (1990).
Dotti, C. G. & Banker, G. A. Experimentally induced alterations in the polarity of developing neurons. Nature 330, 254–256 (1987).
Chalfie, M., Tu, Y., Euskirchen, G., Ward, W. W. & Prasher, D. C. Green fluorescent protein as a marker for gene expression. Science 263, 802–805 (1994).
Nonet, M. L., Saifee, O., Zhao, H., Rand, J. B. & Wei, L. Synaptic transmission deficits in Caenorhabditis elegans synaptobrevin mutants. J. Neurosci. 18, 70–80 (1998).
Jorgensen, E. M. et al. Defective recycling of synaptic vesicles in synaptotagmin mutants of Caenorhabditis elegans. Nature 378, 196–199 (1995).
Sulston, J. E. & Horvitz, H. R. Post-embryonic cell lineages of the nematode Caenorhabditis elegans. Dev. Biol. 56, 110–156 (1977).
Prasher, D. C. & Tsien, R. Y. Wavelength mutations and posttranslational autoxidation of green fluorescent protein. Proc. Natl Acad. Sci. USA 91, 12501–12504 (1994).
Ambros, V. & Moss, E. G. Heterochronic genes and the temporal control of C. elegans development. Trends Genet. 10, 123–127 (1994).
Ruvkun, G. & Giusto, J. The Caenorhabditis elegans heterochronic gene lin-14 encodes a nuclear protein that forms a temporal developmental switch. Nature 338, 313–319 (1989).
Euling, S. & Ambros, V. Heterochronic genes control cell cycle progress and developmental competence of C. elegans vulval precursor cells. Cell 84, 667–676 (1996).
Wightman, B., Bürglin, T. R., Gatto, J., Arasu, P. & Ruvkun, G. Negative regulatory sequences in the lin-14 3′-untranslated region are necessary to generate a temporal switch during Caenorhabditis elegans development. Genes Dev. 5, 1813–1824 (1991).
Wightman, B., Ha, I. & Ruvkun, G. Post-transcriptional regulation of the heterochronic gene lin-14 by lin-4 mediates temporal pattern formation in C. elegans. Cell 75, 855–862 (1993).
Purves, D. & Hadley, R. D. Changes in the dendritic branching of adult mammalian neurones revealed by repeated imaging in situ. Nature 315, 404–406 (1985).
Purves, D. & Lichtman, J. W. Elimination of synapses in the developing nervous system. Science 210, 153–157 (1994).
Wigston, D. J. Repeated in vivo visualization of neuromuscular junctions in adult mouse lateral gastrocnemius. J. Neurosci. 10, 1753–1761 (1990).
Chen, L., Folsom, D. B. & Ko, C.-P. The remodelling of synaptic extracellular matrix and its dynamic relationship with nerve terminals at living frog neuromuscular junctions. J. Neurosci. 11, 2920–2930 (1991).
Bailey, C. H. & Kandel, E. R. Structural changes accompanying memory storage. Annu. Rev. Physiol. 55, 397–426 (1993).
Goodman, C. S. & Shatz, C. J. Developmental mechanisms that generate precise patterns of neuronal connectivity. Cell 72, 77–98 (1993).
Brenner, S. The genetics of Caenorhabditis elegans. Genetics 77, 71–94 (1974).
Clark, S. G., Lu, X-W. & Horvitz, H. R. The Caenorhabditis elegans locus lin-15, a negative regulator of a tyrosine kinase signaling pathway, encodes two different proteins. Genetics 137, 987–997 (1994).
Naito, M., Kohara, Y. & Kurosawa, Y. Identification of a homeobox-containing gene located between lin-45 and unc-24 on chromosome IV in the nematode Caenorhabditis elegans. Nucleic Acids Res. 20, 2967–2969 (1992).
Acknowledgements
We thank M. Nonet for the SNB–GFP DNA; V. Ambros and Y. Hong for lin-14 alleles and lin-14(+) genomic DNA; G. Ruvkun, B. Reinhart and F. Slack for anti-LIN-14 antibodies; and A. Chisholm, A. Zahler, J. Sisson, V. Ambros, M. Nonet, M. Zhen, I. Chin-Sang and members of the Jin and Chisholm labs for helpful discussions and comments. Some strains used in this work were provided by the Caenorhabditis Genetics Center, which is funded by the NIH Center for Research Resources. S.J.H. was supported by a Predoctoral GAANN fellowship. Y.J. is an Alfred P. Sloan Research Fellow.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hallam, S., Jin, Y. lin-14 regulates the timing of synaptic remodelling in Caenorhabditis elegans. Nature 395, 78–82 (1998). https://doi.org/10.1038/25757
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/25757
This article is cited by
-
Spatiotemporal control of a novel synaptic organizer molecule
Nature (2015)
-
Inversion in the worm
Nature (2015)
-
The Caenorhabditis elegans voltage-gated calcium channel subunits UNC-2 and UNC-36 and the calcium-dependent kinase UNC-43/CaMKII regulate neuromuscular junction morphology
Neural Development (2013)
-
MicroRNAs tell an evo–devo story
Nature Reviews Neuroscience (2009)
-
An elegant miRror: microRNAs in stem cells, developmental timing and cancer
Chromosoma (2009)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.