Abstract
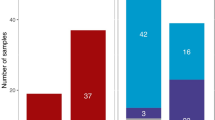
Mitochondrial DNA and the Y chromosome have been used extensively in the study of modern human origins and other phylogenetic questions, but not in the context of their sex-specific modes of transmission. mtDNA is transmitted exclusively by females, whereas the Y chromosome is passed only among males. As a result, differences in the reproductive output or migration rate of males and females will influence the geographic patterns and relative level of genetic diversity on the Y chromosome, autosomes and mtDNA (ref. 1 ). We have found that Y chromosome variants tend to be more localized geographically than those of mtDNA and the autosomes2,5. The fraction of variation within human populations for Y chromosome single nucleotide polymorphisms (SNPs) is 35.5%, versus 80–85% for the autosomes and mtDNA (refs 6, 7, 8 ). A higher female than male migration rate ( via patrilocality, the tendency for a wife to move into her husband's natal household) explains most of this discrepancy, because diverse Y chromosomes would enter a population at a lower rate than mtDNA or the autosomes. Polygyny may also contribute, but the reduction of variation within populations that we measure for the Y chromosome, relative to the autosomes and mitochondrial DNA, is of such magnitude that differences in the effective population sizes of the sexes alone are insufficient to produce the observation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Salem, A.H., Badr, F.M., Gaballah, M.F. & Paabo, S. The genetics of traditional living: Y-chromosomal and mitochondrial lineages in the Sinai Peninsula. Am. J. Hum. Genet. 59, 741–743 (1996).
Seielstad, M.T. et al. Construction of human Y-chromosomal haplotypes using a new polymorphic A to G transition. Hum. Mol. Genet. 3, 2159–2161 (1994).
Ruiz-Linares, A. et al. Geographic clustering of human Y-chromosome haplotypes. Ann. Hum. Genet. 60, 401– 408 (1996).
Underhill, P.A., Jin, L., Zemans, R., Oefner, P.J. & Cavalli-Sforza, L.L. A pre-Columbian Y chromosome-specific transition and its implications for human evolutionary history. Proc. Natl Acad. Sci. USA 93, 196–200 (1996).
Underhill, P.A. et al. Detection of numerous Y chromosome biallelic polymorphisms by denaturing high-performance liquid chromatography. Genome Res. 7, 996–1005 (1997).
Lewontin, R.C. The apportionment of human diversity. Evol. Biol. 6, 381–398 (1972).
Barbujani, G., Magagni, A., Minch, E. & Cavalli-Sforza, L.L. An apportionment of human DNA diversity. Proc. Natl Acad. Sci. USA 94, 4516–4519 (1997).
Excoffier, L., Smouse, P.E. & Quattro, J.M. Analysis of molecular variance inferred from metric distances among DNA haplotypes: application to human mitochondrial DNA restriction data. Genetics 131, 479– 491 (1992).
Cavalli-Sforza, L.L. & Bodmer, W.F. The Genetics of Human Populations (Freeman, San Francisco, 1971).
Miyata, T. et al. Molecular clock of silent substitution: at least six-fold preponderance of silent changes in mitochondrial genes over those in nuclear genes. J. Mol. Evol. 19, 28– 35 (1982).
Hammer, M.F. A recent common ancestry for human Y chromosomes. Nature 378, 376–378 (1995).
Wijsman, E.M. & Cavalli-Sforza, L.L. Migration and genetic population structure with special reference to humans. Annu. Rev. Ecol. Syst. 15, 279–301 (1984).
Slatkin, M. A measure of population subdivision based on microsatellite allele frequencies. Genetics 139, 457–462 (1995).
Feldman, M.W., Kumm, J. & Pritchard, J.K. Mutation and migration in models of microsatellite evolution. in Microsatellites, Evolution and Applications (eds Goldstein, D.J. & Schloetterer, C.) (Oxford University Press, Oxford, in press).
Thomas, J.M.C. Les Ngbaka de la Lobaye (Mouton, The Hague, 1963).
White, D.R. Rethinking polygyny. Curr. Anthropol. 29, 529–572 (1988).
Dorjahn, V. R. in Continuity and Change in African Culture (eds Herskovits, M.J. & Bascomb, W.R.) 87–112 (University of Chicago Press, Chicago, 1959).
Hrdlicka, A. Fecundity in Sioux women. Am. J. Phys. Anthropol. 16, 81–90 (1931).
Isaac, B. Female fertility and marital form among the Mende of rural Upper Bambara Chiefdom, Sierra Leone. Ethnology 19, 297– 313 (1980).
Hewlett, B., van de Koppel, J.M.H. & Cavalli-Sforza, L.L. Exploration ranges of Aka Pygmies of the Central African Republic. Man 17, 418–430 (1982).
Ember, C.R. Myths about hunter-gatherers. Ethnology 17, 439–448 (1978).
Burton, M.L., Moore, C.C., Whiting, J.W.M. & Romney, A.K. Regions based on social structure. Curr. Anthropol. 37, 87–123 (1996).
Murdock, G. P. Ethnographic Atlas (University of Pittsburgh Press, Pittsburgh, 1967).
Melnick, D.J. & Hoelzer, G.A. Differences in male and female macaque dispersal lead to contrasting distributions of nuclear and mitochondrial DNA variation. Int. J. Primatol. 13, 379–393 (1992).
Keane, B., Dittus, W.P.J. & Melnick, D.J. Paternity assessment in wild groups of toque macaques Macaca sinica at Polonnaruwa, Sri Lanka using molecular markers. Mol. Ecol. 6, 267–282 (1997).
Miller, S.A., Dykes, D.D. & Polesky, H.F. A simple salting out procedure for extracting DNA from human nucleated cells. Nuceic Acids Res. 16, 1215 (1988).
Schneider, S., Kueffer, J. M., Roessli, D. & Excoffier, L. Arlequin ver. 1.1, University of Geneva (1997).
Semino, O., Passarino, G., Brega, A., Fellous, M. & Santachiara-Benerecetti, A.S. A view of the Neolithic demic diffusion in Europe through two Y chromosome-specific markers. Am. J. Hum. Genet. 59, 964–968 (1996).
Richards, M. et al. Paleolithic and Neolithic lineages in the European mitochondrial gene pool. Am. J. Hum. Genet. 59, 185– 203 (1996).
Cavalli-Sforza, L.L., Menozzi, P. & Piazza, A. The History and Geography of Human Genes (Princeton University Press, Princeton, New Jersey, 1994).
Acknowledgements
We thank all the DNA donors who participated in this project. Laboratory work was funded by NIH grant GM28428 to L.L.C.-S. The collection of DNA samples in Ethiopia, Sudan and Mali was supported by the Arthur Green Fund of Harvard University and the L.S.B. Leakey Foundation, with the assistance of E. Bekele, M. Ibrahim, M. Traoré and A. Touré. M.T.S. was a U.S. National Science Foundation Predoctoral Fellow. We thank M. Feldman for helpful advice and discussion.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Seielstad, M., Minch, E. & Cavalli-Sforza, L. Genetic evidence for a higher female migration rate in humans. Nat Genet 20, 278–280 (1998). https://doi.org/10.1038/3088
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/3088
This article is cited by
-
Mother-in-Law Daughter-in-Law Conflict: an Evolutionary Perspective and Report of Empirical Data from the USA
Evolutionary Psychological Science (2022)
-
New insights on intercontinental origins of paternal lineages in Northeast Brazil
BMC Evolutionary Biology (2020)
-
The Early Peopling of the Philippines based on mtDNA
Scientific Reports (2020)
-
Sex-biased patterns shaped the genetic history of Roma
Scientific Reports (2020)
-
The paternal and maternal genetic history of Vietnamese populations
European Journal of Human Genetics (2020)