Abstract
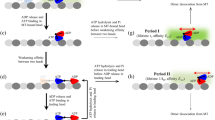
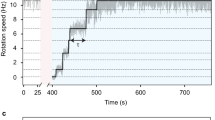
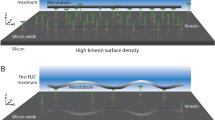
Motor proteins such as kinesin, myosin and polymerase convert chemical energy into work through a cycle that involves nucleotide hydrolysis. Kinetic rates in the cycle that depend upon load identify transitions at which structural changes, such as power strokes or diffusive motions, are likely to occur. Here we show, by modelling data obtained with a molecular force clamp, that kinesin mechanochemistry can be characterized by a mechanism in which a load-dependent isomerization follows ATP binding. This model quantitatively accounts for velocity data over a wide range of loads and ATP levels, and indicates that movement may be accomplished through two sequential 4-nm substeps. Similar considerations account for kinesin processivity, which is found to obey a load-dependent Michaelis–Menten relationship.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Howard, J. Molecular motors: structural adaptations to cellular functions. Nature 389, 561–567 (1997).
Svoboda, K., Schmidt, C. F., Schnapp, B. J. & Block, S. M. Direct observation of kinesin stepping by optical trapping interferometry. Nature 365, 721–727 (1993).
Schnitzer, M. J. & Block, S. M. Kinesin hydrolyses one ATP per 8-nm step. Nature 388, 386–390 (1997).
Howard, J., Hudspeth, A. J. & Vale, R. D. Movement of microtubules by single kinesin molecules. Nature 342, 154–158 (1989).
Visscher, K., Schnitzer, M. J. & Block, S. M. Single kinesin molecules studied with a molecular force clamp. Nature 400, 184–189 (1999).
Svoboda, K. & Block, S. M. Force and velocity measured for single kinesin molecules. Cell 77, 773–784 (1994).
Meyhöfer, E. & Howard, J. The force generated by a single kinesin molecule against an elastic load. Proc. Natl Acad. Sci. USA 92, 574–578 (1995).
Coppin, C. M., Pierce, D. W., Hsu, L. & Vale, R. D. The load dependence of kinesin's mechanical cycle. Proc. Natl Acad. Sci. USA 94, 8539–8544 (1997).
Kojima, H., Muto, E., Higuchi, H. & Yanagida, T. Mechanics of single kinesin molecules measured by optical trapping nanometry. Biophys. J. 73, 2012–2022 (1997).
Peskin, C. & Oster, G. Coordinated hydrolysis explains the mechanical behavior of kinesin. Biophys. J. 68, 202s–211s (1995).
Duke, T. & Leibler, S. Motor protein mechanics: a stochastic model with minimal mechanochemical coupling. Biophys. J. 71, 1235–1247 (1996).
Derényi, I. & Vicsek, T. The kinesin walk: a dynamic model with elastically coupled heads. Proc. Natl Acad. Sci. USA 93, 6775–6779 (1996).
Astumian, R. D. Thermodynamics and kinetics of a brownian motor. Science 276, 917–922 (1997).
Howard, J. The mechanics of force generation by kinesin. Biophys. J. 68, 245s–255s (1995).
Wang, M. D. et al. Force and velocity measured for single molecules of RNA polymerase. Science 282, 902–907 (1998).
Hille, B. Ionic Channels of Excitable Membranes (Sinauer Associates, Sunderland, Massachusetts, 1992).
Ma, Y. Z. & Taylor, E. W. Mechanism of microtubule kinesin ATPase. Biochemistry 34, 13242–13251 (1995).
Ma, Y. Z. & Taylor, E. W. Interacting head mechanism of microtubule-kinesin ATPase. J. Biol. Chem. 272, 724–730 (1997).
Gilbert, S. P., Webb, M. R., Brune, M. & Johnson, K. A. Pathway of processive ATP hydrolysis by kinesin. Nature 373, 671–676 (1995).
Gilbert, S. P., Moyer, M. L. & Johnson, K. A. Alternating site mechanism of the kinesin ATPase. Biochemistry 37, 792–799 (1998).
Moyer, M. L., Gilbert, S. P. & Johnson, K. A. Pathway of ATP hydrolysis by monomeric and dimeric kinesin. Biochemistry 37, 800–813 (1998).
Bagshaw, C. R. Muscle Contraction. (Chapman and Hall, London, 1993).
Hackney, D. D. Evidence for alternating head catalysis by kinesin during microtubule-stimulated ATP hydrolysis. Proc. Natl Acad. Sci. USA 91, 6865–6869 (1994).
Hackney, D. D. Highly processive microtubule-stimulated ATP hydrolysis by dimeric kinesin head domains. Nature 377, 448–450 (1995).
Rice, S. et al. A structural change in the kinesin motor protein that drives motility. Nature 402, 778–784 (1999).
Hua, W., Young, E. C., Fleming, M. L. & Gelles, J. Coupling of kinesin steps to ATP hydrolysis. Nature 388, 390–393 (1997).
Case, R. B., Rice, S., Hart, C. L., Ly, B. & Vale, R. D. Role of the kinesin neck linker and catalytic core in microtubule-based motility. Curr. Biol. 10, 157–160 (2000).
Case, R. B., Pierce, D. W., Hom-Booher, N., Hart, C. L. & Vale, R. D. The directional preference of kinesin motors is specified by an element outside of the motor catalytic domain. Cell 90, 959–966 (1997).
Henningsen, U. & Schliwa, M. Reversal in the direction of movement of a molecular motor. Nature 389, 93–96 (1997).
Endow, S. A. & Waligora, K. W. Determinants of kinesin motor polarity. Science 281, 1200–1202 (1998).
Nishiyama, M. et al. The rising phase of kinesin's 8nm step. Biophys. J. 78, 122A (2000).
Block, S. M., Goldstein, L. S. B. & Schnapp, B. J. Bead movement by single kinesin molecules studied with an optical tweezers. Nature 348, 348–352 (1990).
Vale, R. D. et al. Direct observation of single kinesin molecules moving along microtubules. Nature 380, 451–453 (1996).
Hancock, W. O. & Howard, J. Kinesin's processivity results from mechanical and chemical coordination between the ATP hydrolysis cycles of the two motor domains. Proc. Natl Acad. Sci. USA 96, 13147–13152 (1999).
Cross, R. A., Crevel, I., Carter, N. J., Alonso, M. C., Hirose, K. & Amos, L. A. The conformational cycle of kinesin. Phil. Trans. R. Soc. Lond. B 355, 459–464 (2000).
Finer, J. T., Simmons, R. M. & Spudich, J. A. Single myosin molecule mechanics: piconewton forces and nanometre steps. Nature 368, 113–119 (1994).
Visscher, K. & Block, S. M. Versatile optical traps with feedback control. Methods Enzymol. 298, 460–489 (1998).
Visscher, K., Gross, S. P. & Block, S. M. Construction of multiple-beam optical traps with nanometer-resolution position sensing. IEEE J. Sel. Topics Quant. Elect. 2, 1066–1076 (1996).
Acknowledgements
We thank T. Perkins and L. Satterwhite for helpful discussions, J. de Georgis for squid dissection, and D. Peoples for expert machining. Experiments were carried out at Princeton University and were supported by grants from the NIGMS, NSF and W.M. Keck Foundation (to S.M.B.), predoctoral fellowships from the American Heart Association, the Charlotte Elizabeth Proctor Fund and the Program in Mathematics and Molecular Biology Burroughs Wellcome Fund (to M.J.S.), and a postdoctoral fellowship from the Burroughs Wellcome Fund of the Life Sciences Research Foundation (to K.V.). Data analysis and modelling were also supported by Lucent Technologies.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Schnitzer, M., Visscher, K. & Block, S. Force production by single kinesin motors. Nat Cell Biol 2, 718–723 (2000). https://doi.org/10.1038/35036345
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/35036345
This article is cited by
-
The reaction-diffusion basis of animated patterns in eukaryotic flagella
Nature Communications (2023)
-
Testing pseudotopological and nontopological models for SMC-driven DNA loop extrusion against roadblock-traversal experiments
Scientific Reports (2023)
-
A force-balance model for centrosome positioning and spindle elongation during interphase and anaphase B
Indian Journal of Physics (2022)
-
Force spectroscopy of single cells using atomic force microscopy
Nature Reviews Methods Primers (2021)
-
Macromolecular crowding acts as a physical regulator of intracellular transport
Nature Physics (2020)