Abstract
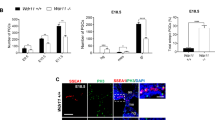
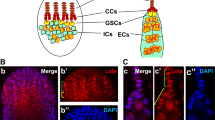
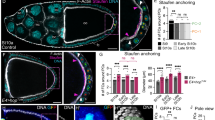
Secreted signalling molecules of the Hedgehog (Hh) family have many essential patterning roles during development of diverse organisms including Drosophila and humans1,2. Although Hedgehog proteins most commonly affect cell fate, they can also stimulate cell proliferation. In humans several distinctive cancers, including basal-cell carcinoma, result from mutations that aberrantly activate Hh signal transduction3. In Drosophila, Hh directly stimulates proliferation of ovarian somatic cells4,5,6. Here we show that Hh acts specifically on stem cells in the Drosophila ovary. These cells cannot proliferate as stem cells in the absence of Hh signalling, whereas excessive Hh signalling produces supernumerary stem cells. We deduce that Hh is a stem-cell factor and suggest that human cancers due to excessive Hh signalling might result from aberrant expansion of stem cell pools.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Ingham, P. W. Transducing Hedgehog: the story so far. EMBO J. 17, 3505–3511 (1998).
Ruiz i Altaba, A. Gli proteins and Hedgehog signaling. Trends Genet. 15, 418–425 (1999).
Wicking, C., Smyth, I. & Bale, A. The hedgehog signalling pathway in tumorigenesis and development. Oncogene 18, 7844–7851 (1999).
Forbes, A. J., Lin, H., Ingham, P. W. & Spradling, A. C. Hedgehog is required for the proliferation and specification of ovarian somatic cells prior to egg chamber formation in Drosophila. Development 122, 1125–1135 (1996).
Forbes, A. J., Spradling, A. C., Ingham, P. W. & Lin, H. The role of segment polarity genes during early oogenesis in Drosophila. Development 122, 3283–3294 (1996).
Zhang, Y. & Kalderon, D. Regulation of cell proliferation and patterning in Drosophila oogenesis by Hedgehog signaling. Development 127, 2165–2176 (2000).
Spradling, A. C. Developmental genetics of oogenesis. In The Development of Drosophila melanogaster (eds Bate, M. & Martinez-Arias, A.) 1–70 (Cold Spring Harbor Lab., Cold Spring Harbor, New York, 1993).
Margolis, J. & Spradling, A. C. Identification and behavior of epithelial stem cells in the Drosophila ovary. Development 121, 3797–3807 (1995).
Hendzel, M. J. et al. Mitosis-specific phosphorylation of histone H3 initiates primarily within pericentromeric heterochromatin during G2 and spreads in an ordered fashion coincident with mitotic chromosome condensation. Chromosoma 106, 348–360 (1997).
Xu, T. & Rubin, G. M. Analysis of genetic mosaics in developing and adult Drosophila tissues. Development 117, 1223–1237 (1993).
Spradling, A. C. et al. The Drosophila germarium: stem cells, germ line cysts and oocytes. Cold Spring Harbor Symp. Quant. Biol. 62, 25–34 (1997).
Harrison, D. & Perrimon, N. A simple and efficient method for generation of marked clones in Drosophila. Curr. Biol. 3, 424–433 (1993).
Price, M. A. & Kalderon, D. Proteolysis of Cubitus interruptus in Drosophila requires phosphorylation by protein kinase A. Development 126, 4331–4339 (1999).
Chiang, C. et al. Essential role for Sonic hedgehog during hair follicle morphogenesis. Dev. Biol. 205, 1–9 (1999).
St-Jacques, B. et al. Sonic hedgehog signaling is essential for hair development. Curr. Biol. 8, 1058–1068 (1998).
Xie, J. et al. Activating smoothened mutations in sporadic basal-cell carcinoma. Nature 391, 90–92 (1998).
Dahmane, N., Lee, J., Robins, P., Heller, P. & Ruiz i Altaba, A. Activation of the transcription factor Gli1 and the Sonic hedgehog signalling pathway in skin tumours. Nature 389, 876–881 (1997).
Gailani, M. R. et al. The role of the human homolog of Drosophila patched in sporadic basal-cell carcinomas. Nature Genet. 14, 78–81 (1996).
Hahn, H. et al. Mutations of the human homolog of Drosophila patched in the nevoid basal cell carcinoma syndrome. Cell 85, 841–851 (1996).
Johnson, R. L. et al. Human homolog of patched, a candidate gene for the basal-cell nevus syndrome. Science 272, 1668–1671 (1996).
Fan, H. R., Oro, A. E., Scott, M. P. & Khavari, P. A. Induction of basal-cell carcinoma features in transgenic human skin expressing sonic hedgehog. Nature Med. 3, 788–792 (1997).
Grachtchouk, M. et al. Basal cell carcinomas in mice overexpressing Gli2 in skin. Nature Genet. 24, 216–217 (2000).
Nilsson, M. et al. Induction of basal cell carcinomas and trichoepitheliomas in mice overexpressing GLI-1. Proc. Natl Acad. Sci. USA 97, 3438–3443 (2000).
Oro, A. E. et al. Basal cell carcinomas in mice overexpressing Sonic hedgehog. Science 276, 817–821 (1997).
Hardy, M. H. The secret life of the hair follicle. Trends Genet. 8, 55–61 (1992).
Rochat, A., Kobayashi, K. & Barrandon, Y. Location of stem cells of human hair follicles by clonal analysis. Cell 76, 1063–1073 (1994).
Taylor, G., Lehner, M. S., Jensen, P. J., Sun, T. -T. & Lavker, R. M. Involvement of follicular stem cells in forming not only the follicle but also the epidermis. Cell 102, 451–461 (2000).
Kruger, K., Blume-Peytavi, U. & Orfanos, C. E. Basal cell carcinoma possibly originates from the outer root sheath and/or the bulge region of the vellus hair follicle. Arch. Dermatol. Res. 291, 253–259 (1999).
Jiang, J. & Struhl, G. Regulation of the Hedgehog and Wingless signalling pathways by the F-box/WD40-repeat protein Slimb. Nature 391, 493–496 (1998).
Methot, N. & Basler, K. Hedgehog controls limb development by regulating the activities of distinct transcriptional activator and repressor forms of Cubitus interruptus. Cell 96, 819–831 (1999).
Acknowledgements
We thank D. Harrison, J. Jiang, N. Methot, N. Patel and A. Spradling for reagents; J. Briscoe for help with confocal microscopy; D. Rabinowitz for statistical modelling of stem cell behaviour; J. Erickson, J. Mohler, M. A. Price and R. Lavker for critical discussions. This work was supported by an NIH grant to D.K.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, Y., Kalderon, D. Hedgehog acts as a somatic stem cell factor in the Drosophila ovary. Nature 410, 599–604 (2001). https://doi.org/10.1038/35069099
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/35069099
This article is cited by
-
Provenance, weathering, and paleoclimatic records of the Pliocene-Pleistocene sequences of the Himalayan foreland basin, NW Himalaya
Arabian Journal of Geosciences (2021)
-
Steady erosion rates in the Himalayas through late Cenozoic climatic changes
Nature Geoscience (2020)
-
Knorpeltumoren: Morphologie, Genetik und Basisaspekte der Targettherapie
Der Pathologe (2020)
-
Serum Extracellular Vesicles Retard H9C2 Cell Senescence by Suppressing miR-34a Expression
Journal of Cardiovascular Translational Research (2019)
-
Spatial correlation bias in late-Cenozoic erosion histories derived from thermochronology
Nature (2018)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.