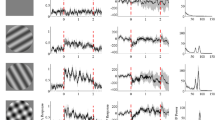
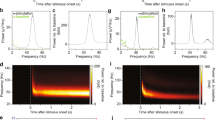
Abstract
VISUAL responses in the retina and the lateral geniculate nucleus (LGN) exhibit oscillatory patterning within a broad range of frequencies1–9. Oscillatory activity is often associated with the synchronization of spatially distributed responses10. Here we demonstrate, with simultaneous multi-electrode recordings from the retina and the LGN, that stationary and moving light stimuli evoke in retinal ganglion cells oscillatory responses in the frequency range of 61 to 114 Hz that become synchronized over distances larger than 20 degrees of visual angle across the nasal and temporal halves of the retina. This temporal patterning of retinal responses is transmitted reliably by LGN neurons, such that stimuli crossing the vertical meridian evoke synchronous responses in the LGNs of both hemispheres. The oscillatory responses are not phase-locked to the stimulus onset, indicating that synchronization results from horizontal interactions in the retina. The occurrence of synchronization depends on global stimulus properties such as size and continuity, suggesting that temporal correlation among responses of spatially segregated ganglion cells can be exploited to convey information relevant for perceptual grouping.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Doty, R. W. & Kimura, D. S. J. Physiol., Lond. 168, 205–218 (1963).
Bishop, P. O., Levick, W. R. & Williams, W. O. J. Physiol., Lond. 170, 598–612 (1964).
Laufer, M. & Verzeano, M. Vision Res. 7, 215–229 (1967).
Arnett, D. W. Expl Brain Res. 24, 111–130 (1975).
Robson, J. G. & Troy, J. B. J. opt. Soc. Am. A4, 2301–2307 (1992).
Ghose, G. M. & Freeman, R. D. J. Neurophysiol. 68, 1558–1574 (1992).
Nuñez, A. K., Amzica, F. & Steriade, M. Neuroscience 51, 269–284 (1992).
Ito, H., Gray, C. M. & Di Frisco, G. V. Soc. Neurosci. Abstr. 20, 61.7(1994).
Wörgötter, F. & Funke, K. Visual Neurosci. 12, 469–484 (1995).
Singer, W. & Gray, C. M. A. Rev. Neurosci. 18, 555–586 (1995).
Perkel, D. H., Gerstein, G. L. & Moore. G. P. Biophys. J. 7, 419–440 (1967).
Gerstein, G. L. & Perkel, D. H. Biophys. J. 12, 453–473 (1972).
Sillito, A. M., Jones, H. E., Gerstein, G. L. & West, D. C. Nature 369, 479–482 (1994).
Stevens, J. K. & Gerstein, G. L. J. Neurophysiol. 39, 239–256 (1976).
Rodieck, R. W. J. Neurophysiol. 30, 1043–1071 (1967).
Arnett, D. W. Expl Brain Res. 32, 49–53 (1978).
Arnett, D. & Spraker, T. E. J. Physiol., Lond 317, 29–47 (1981).
Mastronarde, D. N. Trends Neurosci. 12, 75–80 (1989).
Wässle, H. & Boycott, B. B. Physiol. Rev. 71, 447–480 (1991).
DeVries, S. H. & Baylor, D. A. Cell 10, (suppl.), 1390–1349 (1993).
Vaney, D. I. Prog. retin. Eye Res. 13, 301–355 (1994).
Cook, J. E. & Becker, D. L. Microsc. Res. Tech. 31, 408–419 (1995).
Engel, A. K., König, P., Kreiter, A. K. & Singer, W. Science 252, 1177–1179 (1991).
Engel, A. K., König, P., Gray, C. M. & Singer, W. Eur. J. Neurosci. 2, 588–606 (1990).
König, P. J. Neurosci. Meth. 54, 31–37 (1994).
Peichl, L. & Wässle, H. J. Physiol., Lond. 291, 117–141 (1979).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Neuenschwander, S., Singer, W. Long-range synchronization of oscillatory light responses in the cat retina and lateral geniculate nucleus. Nature 379, 728–733 (1996). https://doi.org/10.1038/379728a0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/379728a0
This article is cited by
-
Portrait of visual cortical circuits for generating neural oscillation dynamics
Cognitive Neurodynamics (2021)
-
Cortical gamma band synchronization through somatostatin interneurons
Nature Neuroscience (2017)
-
Microsaccades enable efficient synchrony-based coding in the retina: a simulation study
Scientific Reports (2016)
-
The oscillation-like activity in bullfrog ON–OFF retinal ganglion cell
Cognitive Neurodynamics (2016)
-
Effect of the small-world structure on encoding performance in the primary visual cortex: an electrophysiological and modeling analysis
Journal of Comparative Physiology A (2015)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.