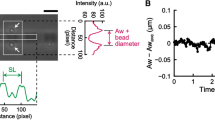
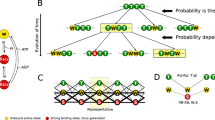
Abstract
The giant muscle protein titin, also called connectin, is responsible for the elasticity of relaxed striated muscle, as well as acting as the molecular scaffold for thick-filament formation1,2. The titin molecule consists largely of tandem domains of the immuno-globulin and fibronectin-III types, together with specialized binding regions and a putative elastic region, the PEVK domain3. We have done mechanical experiments on single molecules of titin to determine their visco-elastic properties, using an optical-tweezers technique. On a fast (0.ls) timescale titin is elastic and force–extension data can be fitted with standard random-coil polymer models, showing that there are two main sources of elasticity: one deriving from the entropy of straightening the molecule; the other consistent with extension of the polypeptide chain in the PEVK region. On a slower timescale and above a certain force threshold, the molecule displays stress-relaxation, which occurs in rapid steps of a few piconewtons, corresponding to yielding of internal structures by about 20 nm. This stress-relaxation probably derives from unfolding of immu-noglobulin and fibronectin domains.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Trinick, J. Cytoskeleton: titin as a scaffold and spring. Curr. Biol. 6, 258–260 (1996).
Maruyama, K. Connectin, an elastic protein of striated muscle. Biophys. Chem. 50, 73–85 (1994).
Labeit, S. & Kolmerer, B. Titins: giant proteins in charge of muscle ultrastructure and elasticity. Science 270, 293–296 (1995).
Linke, W. A. et al. Towards a molecular understanding of the elasticity of titin. J. Mol. Biol. 261, 62–71 (1996).
Gautel, M. & Goulding, D. A molecular map of titin/connectin elasticity reveals two different mechanisms acting in series. FEBS Lett. 385, 11–14 (1996).
Higuchi, H., Nakauchi, Y., Maruyama, K. & Fujime, S. Characterization of α-connectin from striated muscle by dynamic light scattering. Biophys. J. 65, 1906–1915 (1993).
Wang, K., McCarter, R., Wright, J., Beverly, J. & Ramirez-Mitchell, R. Viscoelasticity of the sarcomere matrix of skeletal-muscles: the titin-myosin composite filament is a dual-stage molecular spring. Biophys. J. 64, 1161–1177 (1993).
Soteriou, A., Clarke, A., Martin, S. & Trinick, J. Titin folding energy and elasticity. Proc. R. Soc. Land. B 254, 83–86 (1993).
Erickson, H. P. Reversible unfolding of fibronectin type-III and immunoglobulin domains provides the structural basis for stretch and elasticity of titin and fibronectin. Proc. Natl Acad. Sci. USA 91, 10114–10118 (1994).
Politou, A. S., Thomas, D. J. & Pastore, A. The folding and stability of titin immunoglobulin-like modules, with implications for the mechanism of elasticity. Biophys. J. 69, 2601–2610 (1995).
Flory, P. J. in Statistical Mechanics of Chain Molecules 316–304 (Hanser, Munich, 1989).
Fixman, M. & Kovac, J. Polymer conformational statistics. III. Modified Gaussian models of stiff chains. J. Chem. Phys. 56, 1564–1568 (1973).
Kellermayer, M. S. Z. & Granzier, H. L. Elastic properties of single titin molecules made visible through fluorescent F-actin binding. Biochem. Biophys. Res. Comm. 221, 491–497 (1996).
Linke, W. A., Bartoo, M. I., Ivemeyer, M. & Pollack, G. H. Limits of titin extension in single cardiac myofibrils. J. Muscle Res. Cell Motil. 17, 425–438 (1996).
Politou, A. S., Gautel, M., Pfuhl, M., Labeit, S. & Pastore, A. Immunoglobulin-type domains of titin: same fold, different stability? Biochemistry 33, 4730–4737 (1994).
Fong, S. et al. Structure and stability of an immunoglobulin superfamily domain from twitchin, a muscle protein of the nematode Caenorhabditis elegans. J. Mol. Biol. 264, 624–639 (1996).
Litvinovich, S. V., Novokhatny, V. V., Brew, S. A. & Ingram, K. C. Reversible unfolding of an isolated heparin and DNA binding fragment, the first type III module from fibronectin. Biochim. Biophys. Acta 1119, 57–62 (1992).
Plaxco, K. W., Spitzfaden, C., Campbell, I. D. & Dobson, C. M. Rapid refolding of a proline-rich all-beta-sheet fibronectin type-Ill module. Proc. Natl Acad. Sci. USA 93, 10703–10706 (1996).
Soteriou, A., Gamage, M. & Trinick, J. A survey of the interactions made by the giant protein titin. J. Cell Sci. 104, 119–123 (1993).
Whiting, J., Wardale, J. & Trinick, J. Does titin regulate the length of muscle thick filaments. J. Mol. Biol. 205, 263–268 (1989).
Fürst, D. O., Osborn, M., Nave, R. & Weber, K. The organisation of titin filaments in the half-sarcomere revealed by monoclonal-antibodies in immunoelectron microscopy: a map of ten nonrepetitive epitopes starting at the Z-line extends close to the M-line. J. Cell Biol. 106, 1563–1572 (1988).
Simmons, R. M., Finer, J. T,, Chu, S. & Spudich, J. A. Quantitative measurements of force and displacement using an optical trap. Biophys. J. 70, 1813–1822 (1996).
Bustamante, C. Entropic elasticity of λ-phage DNA. Science 265, 1599–1600 (1994).
Tskhovrebova, L. & Trinick, J. Direct visualization of extensibility in isolated titin molecules. J. Mol. Biol. 265, 100–106 (1997).
Rief, M., Gautel, M., Oesterhelt, F., Fernandez, J. M. & Gaub, H. E. Reversible unfolding of individual titin Ig-domains by AFM. Science (in the press).
Kellermayer, M. S. Z., Smith, S. B., Granzier, H. L. & Bustamante, C. Folding-unfolding transitions in single titin molecules characterized with force-measuring laser tweezers. Science (in the press).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Tskhovrebova, L., Trinick, J., Sleep, J. et al. Elasticity and unfolding of single molecules of the giant muscle protein titin. Nature 387, 308–312 (1997). https://doi.org/10.1038/387308a0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/387308a0
This article is cited by
-
Exploring TTN variants as genetic insights into cardiomyopathy pathogenesis and potential emerging clues to molecular mechanisms in cardiomyopathies
Scientific Reports (2024)
-
Optical tweezers in single-molecule biophysics
Nature Reviews Methods Primers (2021)
-
Protein nanomechanics in biological context
Biophysical Reviews (2021)
-
A HaloTag-TEV genetic cassette for mechanical phenotyping of proteins from tissues
Nature Communications (2020)
-
Single-particle virology
Biophysical Reviews (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.