Short-lived insects surprisingly still suffer senescence under natural conditions.
Abstract
Ageing (senescence) has never been demonstrated convincingly in any insect in the wild, where mean lifespans are probably much shorter than in the laboratory1, and most evidence for senescence in other wild animals (such as mammals) is limited to their reduced survival with age2. Here we show that ageing is detectable in wild populations of a very short-lived insect, the antler fly (Protopiophila litigata), and causes debilitating and costly effects that force a decline not only in survival probability, but also in the reproductive rate of males. Our findings argue against the possibility of a trade-off between fitness components, whereby survival may decline without senescence if investment in reproduction increases with age3, and indicate that ageing rates are subject to intense selection in the wild.
Similar content being viewed by others
Main
Although theory predicts the evolution of rapid senescence in organisms that experience high extrinsic (age-independent) mortality rates4, it has been suggested that very few individuals in these groups (such as insects or small mammals) survive long enough in the wild to exhibit detectable senescence5,6.
We tested for senescence in a wild population of the antler fly, a small dipteran that breeds exclusively on discarded antlers of moose and deer. The tendency of adult flies to spend their lives on a single antler, as well as the long duration of their mating (2.3 h; ref. 7), facilitate the acquisition of field data on mating success and survival. We surveyed mating aggregations on nine moose antlers every 2 h over 72 days, and recorded the presence and mating status (single or coupled) of each of 609 individually marked males8.
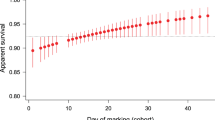
The daily probability of mortality in males increased with age (Fig. 1a; likelihood ratio comparisons with constant model: Gompertz, χ21 = 4.293, P = 0.0383; two-parameter Weibull, χ21 = 3.931, P = 0.0474; three-parameter Weibull, χ21 = 3.950, P > 0.1). The Gompertz model fitted marginally better than the two-parameter Weibull, on the basis of Akaike's information criterion (AIC; Gompertz, 78.11; Weibull, 78.29) and bootstrap analysis (Gompertz chosen in 5,838 of 10,000 iterations).
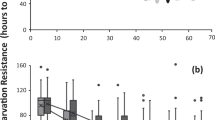
a, Mean daily probability of mortality (hazard rate; circles), h(t), with the fitted Gompertz model (solid blue line) and initial hazard rate (dashed black line; instantaneous ho = 0.1359; γ = 0.0166). b, Mean daily mating success (circles), m(t), with the fitted linear model (solid blue line) and initial mating rate (dashed black line; λ = 0.3741; κ =−0.0076, likelihood ratio P < 0.0001). Dotted green lines indicate 95% confidence limits. Marked males were released at antlers 24–48 h after adult emergence and were presumed to have died on the day after their last sighting, as mean daily sighting probability was high (63%) and the probability of immigration was very low. Mean lifespan, mating success and mating rate did not change over the season (P > 0.2 for all tests). Senescence models were fitted by maximum likelihood, with daily survival and mating rates as random binomial and Poisson processes with age-specific probabilities. Confidence limits were generated by 10,000 bootstraps. Hazard-rate models compared: constant, h(t) = λ; Gompertz, h(t) = hoexp(γt), fitted as Nt+1/Nt = exp[(− ho/γ)(exp(γ(t+1)) − exp(γt))]; three-parameter Weibull, h(t) = ho + αtβ, fitted as Nt+1/Nt = exp[− ho − (α/(β + 1))((t + 1)β + 1 − tβ + 1)]; two-parameter Weibull (ho ≡ 0). Mating-rate models compared: constant; Weibull; linear, m(t) = λ + κt. Because data for the mark–release day were incomplete, these data (and the 75 males that survived for less than 1 day after release) were excluded from the analyses.
The daily mating rate decreased linearly with age (Fig. 1b; linear AIC, 2,337.94; two-parameter Weibull AIC, 2,338.72; constant AIC, 2,345.03). Although the mean mating rate may decline with age because males that mate less frequently live longer, rather than because of senescence, we found no evidence of a trade-off between mean daily mating rate in early life (age, 2–5 days) and lifespan (n = 15 lifespan classes, r2 = 0.05, F = 0.74, P > 0.4). Mating rate is probably a reliable indicator of male reproductive rate in this species, as take-overs are rare and females lay eggs immediately after copulation7. Thus, as predicted by theory4, we found that both survival and reproductive rate declined with age.
To assess the relative fitness costs of senescent declines in survival and reproduction, we compared the reproductive value (that is, the expected lifetime mating success) of males at age 0 days with hypothetical reproductive values based on constant initial (presenescent) daily rates of survival or reproduction. Males lost 1.7 times more fitness as a result of declining reproductive rate than from declining survival rate, but this difference was not significant (bootstrap P = 0.0992).
However, a numerical analysis comparing the relative sensitivity of fitness to small, additive reductions in survival and mating rates at different ages of onset9 indicated that at younger ages (< 20 days), selection was stronger on survival than on mating rate, whereas at older ages (> 20 days), selection was stronger on mating rate than on survival. Net fitness losses from senescence in both traits amounted to about 20% (95% confidence limit: 13–26%) of potential fitness. However, our analysis may underestimate both total fitness losses and relative losses from senescence in reproductive rate if low-quality individuals are under-represented in older age classes10 and if male fertility declines with age11.
We have shown that senescence can be detected in wild insects, despite a high extrinsic mortality rate (about 13% per day) and brief median lifespan (6 days). Because there is a simultaneous decline in both survival and reproduction, our results indicate unambiguously that senescence occurs in this wild insect population. The high net fitness costs of senescence confirm a basic assumption of evolutionary theories — that senescence rates are under strong selection in wild animals.
References
Roach, D. A. Exp. Gerontol. 36, 687–694 (2001).
Promislow, D. E. L. Evolution 45, 1869–1887 (1991).
Partridge, L. & Barton, N. H. Proc. R. Soc. Lond. B 263, 1365–1371 (1996).
Williams, G. C. Evolution 11, 398–411 (1957).
Hayflick, L. Nature 408, 267–269 (2000).
Kirkwood, T. B. L. & Austad, S. N. Nature 408, 233–238 (2000).
Bonduriansky, R. & Brooks, R. J. Can. Entomol. 130, 399–405 (1998).
Bonduriansky, R. & Brooks, R. J. Can. Entomol. 129, 827–830 (1997).
Abrams, P. A. Evol. Ecol. 5, 343–360 (1991).
Service, P. M. Am. Nat. 156, 1–13 (2000).
Prowse, N. & Partridge, L. J. Insect Physiol. 43, 501–512 (1997).
Author information
Authors and Affiliations
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Bonduriansky, R., Brassil, C. Rapid and costly ageing in wild male flies. Nature 420, 377 (2002). https://doi.org/10.1038/420377a
Issue Date:
DOI: https://doi.org/10.1038/420377a
This article is cited by
-
Directional selection coupled with kin selection favors the establishment of senescence
BMC Biology (2023)
-
A natural constant predicts survival to maximum age
Communications Biology (2021)
-
Sexual differences in age-dependent survival and life span of adults in a natural butterfly population
Scientific Reports (2020)
-
Short and fast vs long and slow: age changes courtship in male orb-web spiders (Argiope keyserlingi)
The Science of Nature (2018)
-
Keeping up with the Red Queen: the pace of aging as an adaptation
Biogerontology (2017)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.