Abstract
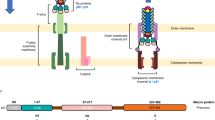
Human adenoviruses1 are responsible for respiratory, gastro-enteric and ocular infections2 and can serve as gene therapy vectors3. They form icosahedral particles with 240 copies of the trimeric hexon protein arranged on the planes and a penton complex at each of the twelve vertices. The penton consists of a pentameric base, implicated in virus internalization4, and a protruding trimeric fibre, responsible for receptor attachment5. The fibres are homo-trimeric proteins containing an amino-terminal penton base attachment domain, a long, thin central shaft and a carboxy-terminal cell attachment or head domain. The shaft domain contains a repeating sequence motif with an invariant glycine or proline and a conserved pattern of hydrophobic residues6. Here we describe the crystal structure at 2.4 Å resolution of a recombinant protein containing the four distal repeats of the adenovirus type 2 fibre shaft plus the receptor-binding head domain. The structure reveals a novel triple β-spiral fibrous fold for the shaft. Implications for folding of fibrous proteins (misfolding of shaft peptides leads to amyloid-like fibrils) and for the design of a new class of artificial, silk-like fibrous materials are discussed.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Schenk,T. in Fields Virology 2111–2148 (Lippincott-Raven, Philadelphia, 1996).
Horwitz,M. S. in Fields Virology 2149–2171 (Lippincott-Raven, Philadelphia, 1996).
Robbins,P. D., Tahara,H. & Ghivizzani,S. C. Viral vectors for gene therapy. Trends Biotechnol. 16, 35–40 (1998).
Wickham,T. J., Mathias,P., Cheresh,D. A. & Nemerow,G. R. Integrins αvβ3 and αvβ5 promote adenovirus internalization but not virus attachment. Cell 73, 309–319 (1993).
Philipson,L., Lonberg-Holm,K. & Petterson,U. Virus-receptor interaction in an adenovirus system. J. Virol. 2, 1064–1075 (1986).
Green,N.M., Wrigley,N. G., Russel,W. C., Martin,S. R. & McLachlan,A. Evidence for a repeating cross β structure in the adenovirus fibre. EMBO J. 2, 1357–1365 (1983).
Chroboczek,J., Ruigrok,R. W. H. & Cusack,S. Adenovirus fiber. Curr. Top. Microbiol. Immunol. 199, 163–200 (1995).
Hong,J. S. & Engler,J. A. Domains required for assembly of adenovirus type 2 fiber trimers. J. Virol. 70, 7071–7078 (1996).
van Raaij,M. J., Louis,N., Chroboczek,J. & Cusack,S. Structure of the human adenovirus serotype 2 fiber head domain at 1.5 Å resolution. Virology 262, 333–343 (1999).
Xia,D., Henry,L. J., Gerard,R. D. & Deisenhofer,J. Crystal structure of the receptor-binding domain of adenovirus type 5 fiber protein at 1.7 Å resolution. Structure 2, 1259–1270 (1994).
Bergelson,J. M. et al. Isolation of a common receptor for coxsackie B viruses and adenoviruses 2 and 5. Science 275, 1320–1323 (1997).
Roelvink,P. W. et al. The coxsackievirus-adenovirus receptor protein can function as a cellular attachment protein for adenovirus serotypes from subgroups A, C, D, E, and F. J. Virol. 72, 7909–7915 (1998).
Stouten,P. F. W., Sander,C., Ruigrok,R. W. H. & Cusack,S. New triple-helical model for the shaft of the adenovirus fibre. J. Mol. Biol. 226, 1073–1084 (1992).
Devaux,C., Adrian,M., Berthet-Colominas,C., Cuasck,S. & Jacrot,B. Structure of adenovirus fibre. I. Analysis of crystals of fibre from adenovirus serotypes 2 and 5 by electron microscopy and X-ray crystallography. J. Mol. Biol. 215, 567–588 (1990).
Louis,N. Etude de la structure de la fibre de l'adénovirus humain de la sérotype 2; son dévenir et son rôle au cours de l'infection. PhD thesis, Univ. Joseph-Fourier, Grenoble (1994).
Mitraki,A. et al. Unfolding studies of human adenovirus type 2 fiber trimers: evidence for a stable domain. Eur. J. Biochem. 264, 599–606 (1999).
Devaux,C., Caillet-Boudin,M. L., Jacrot,B. & Boulanger,P. Crystallization, enzymatic cleavage, and the polarity of the adenovirus type 2 fiber. Virology 161, 121–128 (1987).
Brunger,A. T. et al. Crystallography & NMR System: A new software suite for macromolecular structure determination. Acta Crystallogr. D 54, 905–921 (1998).
Ruigrok,R. W. H., Barge,A., Albiges-Rizo,C. & Dayan,S. Structure of adenovirus fibre. II. Morphology of single fibres. J. Mol. Biol. 215, 589–596 (1990).
Ruigrok,R. W. H., Barge,A., Mittel,S. K. & Jacrot,B. The fibre of bovine adenovirus type 3 is very long but bent. J. Gen. Virol. 75, 2069–2073 (1994).
Hess,M., Cuzange,A., Ruigrok,R. W. H., Chroboczek,J. & Jacrot,B. The avian adenovirus penton: two fibres and one base. J. Mol. Biol. 252, 379–385 (1995).
Beck,B. & Brodsky,B. Supercoiled protein motifs: The collagen triple-helix and the a-helical coiled coil. J. Struct. Biol. 122, 17–29 (1998).
Steinbacher,S. et al. Crystal structure of P22 tailspike protein: interdigitated subunits in a thermostable trimer. Science 265, 383–386 (1994).
Yoder,M. D., Keen,N. T. & Jurnak,F. New domain motif: Structure of pectate lyase C, a secreted plant virulence factor. Science 260, 1503–1507 (1993).
Marsh,R. E., Corey,R. B. & Pauling,L. The structure of Tussah silk fibroin. Acta Crystallogr. 8, 710–715 (1955).
Cerritelli,M. E, Walls,J. S., Simon,M. N., Conway,J. F. & Steven,A. C. Stoichiometry and dominant organization of the long-fiber of bacteriophage T4: A hinged viral adhesion. J. Mol. Biol. 260, 767–780 (1996).
Tao,Y., Strelkov,S. V., Mesyanshinov,V. & Rossmann,M. G. Structure of bacteriophage T4 fibritin: A segmented coiled coil and the role of the C-terminal domain. Structure 5, 789–798 (1997).
Betts,S. & King,J. There's a right and a wrong way: in vivo and in vitro folding, misfolding and subunit assembly of the P22 tailspike. Structure 7, R131–R139 (1999).
O'Brien,J. P. et al. in Silk Polymers, Materials Science and Biotechnology 104–117 (ACS Symposium Series 544, American Chemical Society, Charlottesville, Virginia, 1993).
Collaborative Computational Project Number 4. The CCP4 suite: Programs for protein crystallography. Acta Crystallogr. D 50, 760–763 (1994).
Acknowledgements
We thank P. Goeltz and N. Cohet for help with cloning, expression and purification and J. Gagnon for comments. M.J.v.R. was supported by an EU Biotech II fellowship. The EMBL-ESRF Joint Structural Biology Group provided synchrotron radiation facilities.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
van Raaij, M., Mitraki, A., Lavigne, G. et al. A triple β-spiral in the adenovirus fibre shaft reveals a new structural motif for a fibrous protein. Nature 401, 935–938 (1999). https://doi.org/10.1038/44880
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/44880
This article is cited by
-
Cryo-EM structure of ssDNA bacteriophage ΦCjT23 provides insight into early virus evolution
Nature Communications (2022)
-
Human adenovirus binding to host cell receptors: a structural view
Medical Microbiology and Immunology (2020)
-
Genome Analysis of A Novel Recombinant Human Adenovirus Type 1 in China
Scientific Reports (2019)
-
Comparing proteins and nucleic acids for next-generation biomolecular engineering
Nature Reviews Chemistry (2018)
-
Grass carp reovirus-GD108 fiber protein is involved in cell attachment
Virus Genes (2017)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.