Abstract
Although maternal human immunodeficiency virus type 1 (HIV-1) transmission occurs during gestation, intrapartum and postpartum (by breast-feeding), 50–70% of all infected children seem to acquire HIV-1 shortly before or during delivery1. Epidemiological evidence indicates that mucosal exposure is an important aspect of intrapartum HIV transmission2,3. A simian immunodeficiency virus (SIV) macaque model has been developed4 that mimics the mucosal exposure that can occur during intrapartum HIV-1 transmission. To develop immunoprophylaxis against intrapartum HIV-1 transmission, we used SHIV–vpu+ (refs. 5,6), a chimeric simian–human virus that encodes the env gene of HIV-IIIB. Several combinations of human monoclonal antibodies against HIV-1 have been identified that neutralize SHIV–vpu+ completely in vitro through synergistic interaction7. Here, we treated four pregnant macaques with a triple combination of the human IgG1 monoclonal antibodies F105, 2G12 and 2F5. All four macaques were protected against intravenous SHIV–vpu+ challenge after delivery. The infants received monoclonal antibodies after birth and were challenged orally with SHIV–vpu+ shortly thereafter. We found no evidence of infection in any infant during 6 months of follow-up. This demonstrates that IgG1 monoclonal antibodies protect against mucosal lentivirus challenge in neonates. We conclude that epitopes recognized by the three monoclonal antibodies are important determinants for achieving substantial protection, thus providing a rational basis for AIDS vaccine development.
Similar content being viewed by others
Main
The monoclonal antibody combination used for passive immunization was highly synergistic and targeted to conserved epitopes: F105 (a human IgG1k monoclonal antibody) recognizes a discontinuous epitope that overlaps the CD4 binding domain8; 2G12 (a human IgG1 monoclonal antibody) recognizes a conformationally sensitive, glycosylation-dependent gp120 epitope9; and 2F5 (a human monoclonal antibody against gp41) recognizes the sequence ELDKWA (ref. 10). All three monoclonal antibodies neutralize laboratory and primary HIV isolates7,8,9,10,11, including the primary isolate HIV89.6 (ref. 12) (Table 1). Even though considerably higher concentrations were required to neutralize HIV89.6 by single-agent monoclonal antibodies than for SHIV–vpu+, the triple combination was so synergistic that the total monoclonal antibody dose required for 50% neutralization was the same for HIV89.6 as for SHIV–vpu+ ( Table 1). The triple monoclonal antibody combination also completely neutralized both viruses in human and macaque peripheral blood mononuclear cells (PBMCs) (ref. 7 and data not shown).
Next, we infused a single dose of the triple combination of F105, 2G12 and 2F5 into pregnant macaque L923 5 days before cesarean section ( Fig. 1) and assessed plasma levels serially, using assays specific for each of the three monoclonal antibodies. The dam's plasma, obtained at cesarean section (day 0), contained 52.3 μg/ml F105, 114.9 μg/ml 2G12 and 72.4 μg/ml 2F5, which greatly exceeded the levels required to neutralize SHIV–vpu+ in vitro by single agents or the triple combination (Table 1). The dam's plasma completely neutralized SHIV–vpu+ at a dilution of 1:8 until postnatal day 4, after only one dose of the monoclonal antibody combination ( Fig. 1). Infant PK1, treated with monoclonal antibodies prenatally and twice postnatally on days 0 and 8, maintained 100% virus neutralization at a plasma dilution of 1:8 until at least postnatal day 21. Cord blood of PK1 contained 12.8 μg/ml F105, 12.8 μg/ml 2G12 and 6 μg/ml 2F5, indicating that neutralizing levels had been reached by transport of human IgG1 monoclonal antibodies across the macaque placenta.
The dam (L923; ●), received an intravenous administration of monoclonal antibodies F105, 2G12 and 2F5 5 days before cesarean section on day 0. Daily blood samples were collected for the first postnatal week and weekly thereafter. The infant (PK1; ▪) received an intravenous dose of the three monoclonal antibodies on day 0 and again on day 8. Because of its small size, only limited blood samples could be collected. The plasma samples of mother and infant were tested for their ability to neutralize SHIV–vpu+ in an MT-2 assay. Data with 1:8 plasma dilutions are shown.
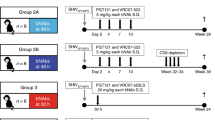
After the initial pharmacokinetic studies, we infused pregnant macaque dams L605, L943, J421 and L975 with the triple monoclonal antibody combination 5 days before cesarean section and again 3 days postpartum ( Fig. 2a). We challenged the dams with intravenous SHIV–vpu+ 1 hour after the second infusion. We detected neutralizing levels of the three monoclonal antibodies in plasma of all dams ( Fig. 2c, e and g). The monoclonal antibodies had the following mean plasma half-lives: F105, 7.2 ± 2.2 days; 2G12, 14.0 ± 7.9 days; 2F5, 4.2 ± 0.8 days, comparable to published data13,14,15. Serial blood samples remained negative for SHIV–vpu+ by PBMC co-culture, DNA PCR (Table 2), RT–PCR (not shown) and serology, using either SIV or HIV antigens ( Fig. 3a, c, and e). Necropsy tissues (PBMCs, mandibular, mesenteric and inguinal lymph nodes, spleen and thymus) obtained 6 months after challenge were negative for infection by DNA PCR (using 1 μg genomic DNA) and co-cultivation (using between 1 × 106 and 1 × 107 cells) (not shown). In contrast, all control juvenile/adult macaques (R1, R3, R4, R5 and L927) became infected (P=0.016 ) and tested positive by RT-PCR during peak viremia (not shown) and by DNA PCR, virus isolation (Table 2) and serology (Fig. 3a, c, and e). Systemic infection was confirmed in lymphoid tissues at necropsy by DNA PCR in all positive controls (not shown).
a and b, Time course of intravenous (i.v.) infusion of the triple monoclonal antibody (mAb) combination, cesarean section delivery and SHIV–vpu+ inoculation of rhesus macaque dams ( a) and their infants (b). c–h, Pharmacokinetics of monoclonal antibodies F105 (c and d), 2G12 (e and f) and 2F5 (g and h) in dams (c,e,g) and neonates (d,f,h). Symbols (keys) identify different macaques. Plasma samples collected from the dams before the first monoclonal antibody infusion (d-5; sample a, b, c, e and g) contained no detectable monoclonal antibodies. Plasma samples were collected from the dams during cesarean section (d0) and on day 3 (d3) before the second monoclonal antibody infusion (trough, t). Peak levels were determined 30 min after this infusion (p; c, e and g). SHIV–vpu+ challenge followed 1 h after the completion of the monoclonal antibody infusion. Because of their small size, only cord blood (c; b, d, f and h) and one set of samples before the third monoclonal antibody infusion on day 8 (d8; e) could be collected from the infants challenged orally (p.o.) with SHIV–vpu+ 1–4 h after the completion of the second monoclonal antibody infusion.
a – d, Plasma samples from adults (a and c) and infants (b and d). a and b, Western blot analysis with HIV-2 strips routinely used to monitor anti-SIV antibody responses. c and d, Western blot analysis with HIV-1 strips that could be used because the human monoclonal antibodies had been eliminated from the monkeys plasma by day 120 (Fig. 2). Left margins, migration of viral proteins; arrows (right margins), migration of Gag antigen (a and b) or envelope glycoproteins (c and d). e and f, Antibody responses detected by anti-SIV Gag ELISA in plasma samples from adults (e) and infants (f). O, samples collected before challenge on day 0; N, plasma samples collected on the day of necropsy, 6 months after challenge. Macaque names, above blots and below graphs.
Oral exposure of neonatal macaques to cell-free SIV or SHIV–vpu+ results in reproducible systemic infection4,17. We administered the three monoclonal antibodies intravenously to the four pregnant dams described above (Fig. 2a), delivered their offspring by cesarean section 5 days later, and then infused each infant intravenously with the three monoclonal antibodies (Fig. 2b. Then, 1–4 hours later, we challenged the neonates orally with SHIV–vpu+. At 8 days after this exposure, we administered a final monoclonal antibody infusion to each neonate (Fig. 2b). As controls, we challenged four untreated neonates (R9C, R10C, R11C and R16C) orally with SHIV–vpu+.
We detected neutralizing levels of each monoclonal antibody in cord blood samples. High levels of each monoclonal antibody were achieved in plasma obtained from each treated infant subsequently (Fig. 2d, f and h). During the next 6 months, we evaluated both groups of infants for evidence of infection. Each untreated neonate was positive for virus isolation throughout (Table 3). In contrast, the infants treated with monoclonal antibody (R12M, R13M, R14M, and R15M) were protected (P=0.028). In these treated infants, we could not detect any plasma viral RNA during the time of peak viremia in untreated infants (not shown). PBMC co-cultures and DNA PCR of the four infants treated with monoclonal antibody remained negative throughout ( Table 3). Serology at 6 months after challenge was negative (Fig. 3b, d and f). Furthermore, we found no evidence of infection in lymphoid tissues or plasma obtained at necropsy (Table 4).
As we found no evidence of infection by any test, our data indicate that the triple combination of human IgG1 monoclonal antibodies protected all macaques against either intravenous or mucosal challenge. We accomplished these ‘proof-of-concept’ experiments with SHIV–vpu+, a chimeric virus that encodes env of the laboratory-adapted HIV-IIIB known to be sensitive to neutralization. Because the triple combination we studied in vivo was highly synergistic and also completely neutralized the primary isolate HIV89.6 in human PBMCs, this or similar combination regimens also hold promise for protection against primary isolates.
Encouraging data have been obtained in recent passive immunization experiments. Although monoclonal antibody 2F5 used singly did not prevent infection after intravenous HIV-1 challenge in chimpanzees, even though plasma 2F5 levels at the time of challenge were more than twice those in the adult macaques in our study, peak viremia was delayed in some or significantly decreased in other animals13. More recently, 2F5 and 2G12 were infused in combination with immunoglobins against HIV (HIVIG) before intravenous challenge of adult rhesus macaques with the pathogenic SHIV–89.6PD variant15; this combination prevented infection in some macaques and decreased virus burdens in the others. Direct comparisons with these and other studies are difficult to make, as either route of virus exposure, virus strain (and thus sensitivity to neutralization) or choice of antibodies differ. Moreover, a wide spectrum of pathogenicity exists among viral strains, including the various SHIVs generated from different HIV-1 clade B clones. In general, HIV-1 challenge of chimpanzees is characterized by low-level replication without disease. In contrast, infection of macaques with SHIV89.6PD (ref. 15) or SHIVDH12 (ref. 18 ) results in CD4+ T-cell depletion. Similarly, two SHIV variants derived from the HXBc2 molecular clone of the laboratory-adapted HIV-IIIB also vary in pathogenicity. Although no disease was reported in SHIV–vpu+-infected adult macaques5,6, one macaque inoculated as neonate with SHIV–vpu+ has developed persistent inversion of CD4+/CD8+ T-cell ratios, depletion of CD4+CD29+ T-cell subsets, and depletion of CD4+ T cells to less than 300 cells/μl after 4 years of observation17, consistent with the belief that the variant used in our studies has low pathogenicity. In contrast, SHIVKU-1, a variant derived by serial passage of this strain, is virulent after oral, intravenous or vaginal exposure19. Passively administered polyclonal neutralizing serum or serum from naive macaques did not protect macaques against oral SHIVKU-1 challenge19. Passive transfer of ‘pooled’, unfractionated serum from chronically SIV-infected rhesus macaques has been shown to protect rhesus macaques from oral exposure to cell-free SIVmac251, but control serum from naive macaques did not. Unexpectedly, the passively administered protective serum had no detectable neutralizing activity against the challenge virus in vitro but contained IgG, IgA and IgM, in addition to high levels of chemokines20. Consequently, the protective component(s) could not be established. In contrast, our study used only well-characterized, neutralizing human IgG1 monoclonal antibodies, and provides evidence that secretory IgA or cytokines are not necessary for protection from mucosal lentivirus exposure, even though it has been generally believed that mucosal immunity, particularly secretory IgA, is required to protect against mucosal virus exposure.
The mechanism involved in the protection against mucosal SHIV–vpu+ is unknown. The triple combination of F105, 2G12 and 2F5 could have protected simply by blocking or altering virus binding to cell surface CD4 and chemokine co-receptors. However, it is possible that the challenge virus SHIV–vpu+ was not effectively neutralized by saliva, crossed the mucosal barrier by mechanisms that are unknown, and infected the first set of target cells. In neutralization studies, saliva samples collected from the monoclonal antibody-treated infants before virus challenge were not more effective than control saliva samples collected from naive infants (not shown).
Human IgG1 can facilitate other protective mechanisms, such as complement activation or deposition, which could directly lyse virions or infected cells21, and antibody-dependent cell-mediated cytotoxicity22. Potent antibody-dependent cell-mediated cytotoxicity activity of monoclonal antibodies 2G12 and F105 has been found in vitro9,22. These mechanisms could maintain the viral load well below our level of detection until additional virus-specific, MHC class I-restricted, cellular immune host defenses could develop and eliminate a small number of cells infected when the virus initially crossed the mucosal surface. The high level of neutralizing monoclonal antibodies would have prevented further ‘waves’ of viral spread from these initial target cells. At present, we cannot discount this possibility; however, using sensitive tissue culture, molecular and immunologic methods of detection, it seems that solid protection, as defined by the threshold of detection for our assays, was achieved. No virus could be detected either by sensitive co-culture or DNA PCR methods in any of the infants treated with monoclonal antibody that were challenged orally. Furthermore, no evidence of a humoral antibody response to infection (that is, no seroconversion) was detected in any of the monoclonal antibody recipients. Clearly, none of the macaques treated with monoclonal antibody was persistently infected, even if the possibility of low-level, transient local infection at the level of the mucosa cannot be ruled out.
In conclusion, we have demonstrated that passive immunity, consisting solely of well-defined, broadly neutralizing human IgG1 monoclonal antibodies, can protect primates against both parenteral and mucosal lentivirus exposure. We conclude that the broadly neutralizing human monoclonal antibodies used in our study react with three epitopes that are essential determinants for achieving protection. Efforts to understand how these human IgG1 antibodies protect against mucosal lentivirus infection could yield new insights and provide focus for future efforts directed at the rational development of a safe, effective AIDS vaccine. Furthermore, our passive immunoprophylaxis approach may represent a new approach for preventing mother-to-infant transmission of HIV.
Methods
Animals.
Rhesus monkeys (Macaca mulatta) from a specific pathogen-free colony were used according to National Institutes of Health Guidelines on the Care and Use of Laboratory Animals at The University of Texas, M. D. Anderson Cancer Center, a facility fully accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International. The experiments were approved by the Animal Care and Use Committees at The University of Texas, M.D. Anderson Cancer Center and at the Dana-Farber Cancer Institute. Fetal ultrasound determinations and cesarean section deliveries were done on anesthetized macaques as described4.
Administration of human monoclonal antibodies.
All monoclonal antibody preparations were of clinical grade purity and were endotoxin-free. Polymune Scientific produced monoclonal antibodies 2G12 and 2F5. The macaques (dams or neonates) were treated intravenously with 10 mg/kg of each of the three monoclonal antibodies in combination.
Virus stocks, detection of virus infection of SHIV–vpu+-exposed macaques, and statistical analysis.
Both SHIV–vpu+ virus stocks used for either oral or intravenous challenge were propagated in rhesus monkey PBMC cultures with human interleukin (IL-2). Supernatants were clarified by centrifugation, filtered, and stored in vapor-phase liquid nitrogen. Both SHIV–vpu+ stocks were titrated in CEMx174 cells. The oral SHIV–vpu+ stock contained 2.078 × 105 50% tissue culture infectious doses (TCID50)/ml. To determine the 50% animal infectious dose (AID50) for the oral route, seven neonatal rhesus monkeys were exposed orally to serial dilutions of cell-free SHIV–vpu+. This virus stock contained 3.79 oral AID50/ml (ref. 23). For oral challenge, 10 AID50 (determined for the oral route in neonates) were given in a non-traumatic manner. This dose yields a 99% probability of infection23.
The intravenous SHIV–vpu+ stock contains 4,600 TCID50/ml and 4,600 intravenous AID50/ml (ref. 6). Therefore, the intravenous SHIV–vpu+ stock contains 1,214-fold more AID50/ml yet 45-fold less TCID50/ml than the oral SHIV–vpu+ stock. These results are consistent with the fact that the intravenous route of exposure requires considerably less virus than the oral or other mucosal routes24. All intravenous challenges used 10 AID50 as determined by intravenous titration in juvenile macaques6. This dose yields a 99% chance of systemic infection23.
Methods for PBMC cell culture, DNA PCR, and QC RT–PCR have been described4,16. Necropsy samples from lymphoid organs were minced. Cells were layered onto Ficoll and centrifuged to obtain the mononuclear cells. Cells were co-cultivated with CEMx174 cells and titrated by end-point dilution in quadruplicate wells. Culture supernatants were analyzed for p27 antigen as described4. Plasma samples were analyzed with commercially available western blot strips prepared from HIV-2 antigens as described4. HIV-1 western blot analyses were done using kits (Epitope, Beaverton, Oregon) according to the manufacturer's instructions. Statistically significant differences between the positive control and the monoclonal antibody treatment groups were determined by Fisher's exact test.
Anti-SIV Gag enzyme-linked immunosorbent assay (ELISA).
96-well plates were coated overnight at room temperature with recombinant Gag p27 SIVmac251 (ImunoDiagnostics, Bedford, Massachusetts) (3 μg/ml 0.05 M Na2CO3, pH 9.6), washed three times in PTA buffer (0.05 M K2HPO4/KH2PO4, 0.1% Tween 20, 0.02% sodium azide, pH 7.4) and blocked at room temperature for 1 h with 0.2% nonfat dry milk in PTA buffer. Then the plates were incubated at room temperature for 2 h with plasma samples (1:50 dilution), washed three times as described above, and incubated at 4 °C overnight with a conjugate of antibody against monkey IgG and alkaline phosphatase (Sigma) and washed as described above. Alkaline phosphatase activity was detected using a p-nitrophenyl phosphate system (Sigma). Absorbance was measured after 50 min of incubation with reconstituted p-nitrophenyl phosphate at a wavelength of 410 nm. Each sample was measured in triplicate. Data represent mean values.
Plasma monoclonal antibody concentration and pharmacokinetic analysis.
Assays to quantify levels of F105, 2G12 and 2F5 have been described15,25. Anti-idiotype, ELISA-based assays were used to measure monoclonal antibodies F105 and 2G12. A peptide-binding assay was used to quantify monoclonal antibody 2F5. To determine the half-lives of the three monoclonal antibodies, natural logs of plasma monoclonal antibody levels were plotted as a function of time from the end of infusion. Slopes (m) of the linear graphs were determined by least-squares analysis. Correlation coefficients (R2) ranged from 0.981 to 0.9996. Half-lives were calculated as t1/2=−(ln 2)/m.
Saliva collection and virus neutralization assay.
Saliva was collected from the oral cavities of neonatal macaques using Weck-cel sponges (Xomed Surgical Products, Jacksonville, Florida). Each sponge was placed in 200 μl elution buffer containing 10 mM phosphate-buffered saline, protease inhibitor, bestatin, 100 U/ml penicillin/streptomycin, 100 μg/ml gentamicin sulfate and 600 U/ml mycostatin. Sponges were centrifuged in 0.45-μm centrifuge filters (Fisher Scientific) at 15,000g for 10 min at 4 °C. Neutralization activity was compared in saliva samples obtained at birth from neonates treated with monoclonal antibody or untreated control neonates. Serial dilutions of salivary filtrate were assayed in duplicate, as measured in a standard MT-2 cell infection assay with SHIV–vpu+ (ref. 7).
References
Mofenson, L. & Wilfert, C. in Pediatric AIDS; The Challenge of HIV Infection in Infants, Children and Adolescents 3rd edn. (eds. Pizzo, P.A. & Wilfert, C.M.) 487–513 (Williams & Wilkins, Baltimore, Maryland, 1998).
Rogers, M.F. Reducing the risk of maternal-infant transmission of HIV by attacking the virus. N. Engl. J. Med. 341, 441– 443 (1999).
Williams-Herman, D., et al. Risk factors for perinatal HIV-1 transmission. Abstract PO-B05-1064 (International Conference on AIDS, 6–11 June 1993).
Baba T.W. et al. Mucosal infection of neonatal rhesus monkeys with cell-free SIV . AIDS Res. Hum. Retroviruses 10, 351– 357 (1994).
Li, J. et al. Persistent infection of macaques with simian-human immunodeficiency viruses. J. Virol. 69, 7061– 7071 (1995).
Lu, Y. et al. Utility of SHIV for testing HIV-1 vaccine candidates in macaques . J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 12, 99–106 (1996).
Li, A. et al. Synergistic neutralization of simian-human immunodeficiency virus SHIV-vpu+ by triple and quadruple combinations of human monoclonal antibodies and high-titer anti-human immunodeficiency virus type 1 immunoglobulins . J. Virol. 72, 3235–3240 (1998).
Posner M.R., et al. An IgG human monoclonal antibody that reacts with HIV 1 gp120, inhibits virus binding to cell, and neutralized infection. J. Immunol. 146, 4325–4332 ( 1991).
Trkola, A. et al. Human monoclonal antibody 2G12 defines a distinctive neutralization epitope on the gp120 glycoprotein of human immunodeficiency virus type 1. J. Virol. 70, 1100–1108 (1996).
Muster T., et al. A conserved neutralizing epitope on gp41 of human immunodeficiency virus type 1. J. Virol. 67, 6642– 6647 (1993).
Mascola J.R., et al. Potent and synergistic neutralization of human immunodeficiency virus (HIV) type 1 primary isolates by hyperimmune anti-HIV immunoglobulin combined with monoclonal antibodies 2F5 and 2G12. J. Virol. 71, 7198–7206 (1997).
Collman, R. et al. An infectious molecular clone of an unusual macrophage-tropic and highly cytopathic strain of human immunodeficiency virus type 1. J. Virol. 66, 7517–7521 (1992).
Conley A.J. et al. The consequence of passive administration of an anti-human immunodeficiency virus type 1 neutralizing monoclonal antibody before challenge of chimpanzees with a primary virus isolate. J. Virol. 70, 6751–6758 (1996).
Posner, M.R . Cavacini L.A., Emes, C.L., Power, J. & Byrn, R.A. Neutralization of HIV-1 by F105, a human monoclonal antibody to the CD4 binding site of gp120. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 6, 7–14 (1993).
Mascola, J.R. et al. Protection of macaques against pathogenic simian/human immunodeficiency virus 89.6PD by passive transfer of neutralizing antibodies. J. Virol. 73, 4009–4018 ( 1999).
Liska, V. et al. Viremia and AIDS in rhesus macaques after intramuscular inoculation of plasmid DNA encoding full-length SIVmac239. AIDS Res. Hum. Retroviruses 15, 445–450 ( 1999).
Ruprecht, R.M. et al. Oral transmission of primate lentiviruses. J. Infect. Dis. 179, S408–412 ( 1999).
Shibata, R. et al. Neutralizing antibody directed against the HIV-1 envelope glycoprotein can completely block HIV-1/SIV chimeric virus infections of macaque monkeys. Nature Med. 5, 204– 210 (1999).
Joag, S.V. et al. Passively administered neutralizing serum that protected macaques against infection with parenterally inoculated pathogenic simian-human immunodeficiency virus failed to protect against mucosally inoculated virus. AIDS Res. Hum. Retroviruses 15, 391–394 (1999).
Van Rompay, K.K. et al. Passive immunization of newborn rhesus macaques prevents oral simian immunodeficiency virus infection. J. Infect. Dis. 177, 1247–1259 (1998).
Sullivan, B.L. et al. Susceptibility of HIV-1 plasma virus to complement-mediated lysis. Evidence for a role in clearance of virus in vivo. J. Immunol. 157, 1791–1798 (1996).
Posner, M.R., Elboim, H.S., Cannon, T., Cavacini, L. & Hideshima, T. Functional activity of an HIV-1 neutralizing IgG monoclonal antibody: ADCC and complement mediated lysis. AIDS Res. Hum. Retroviruses 8, 553–558 ( 1992).
Spouge, J.L. Statistical analysis of sparse infection data and its implications for retroviral trials in primates. Proc. Natl. Acad. Sci. USA 89, 7581–7585 (1992).
Baba, T.W. et al. Infection and AIDS in adult macaques after nontraumatic oral exposure to cell-free SIV. Science 272, 1486–1489 (1996).
Cavacini L.A. et al. Plasma pharmacokinetics and biological activity of a HIV-1 neutralizing human monoclonal antibody, F105, in cynomolgous monkeys. J. Immunotherapy 15, 251–256 (1994)
Acknowledgements
We thank C. Gallegos, B. Odlum and S. Sharp for the preparation of this manuscript. This work was supported in part by National Institutes of Health grants RO1 AI34266 and RO1 AI32330 to R.M.R. and RO1 AI26926 to L.A.C. and M.R.P. It was also supported by the Pediatric AIDS Foundation grant 50864PG23 to R.M.R. and by the Center for AIDS Research core grant IP30 28691 awarded to the Dana-Farber Cancer Institute as support for the Institute's AIDS research efforts. T.W.B. was a recipient of an NIH Clinical Investigator Development Award (KO8-AI01327). R.H-L. was supported by a grant from the Swiss National Science Foundation (fellowship number 823A-50315).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Baba, T., Liska, V., Hofmann-Lehmann, R. et al. Human neutralizing monoclonal antibodies of the IgG1 subtype protect against mucosal simian–human immunodeficiency virus infection. Nat Med 6, 200–206 (2000). https://doi.org/10.1038/72309
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/72309
This article is cited by
-
Fabrication of TPGS decorated Etravirine loaded lipidic nanocarriers as a neoteric oral bioavailability enhancer for lymphatic targeting
Discover Nano (2024)
-
Expression of mammalian proteins for diagnostics and therapeutics: a review
Molecular Biology Reports (2022)
-
IgG-like bispecific antibodies with potent and synergistic neutralization against circulating SARS-CoV-2 variants of concern
Nature Communications (2022)
-
Broadly neutralizing antibodies and vaccine design against HIV-1 infection
Frontiers of Medicine (2020)
-
Engineering and characterising a novel, highly potent bispecific antibody iMab-CAP256 that targets HIV-1
Retrovirology (2019)