Abstract
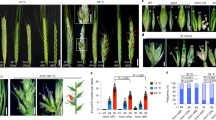
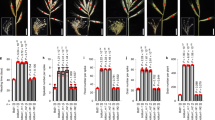
The domestication of cereal crops such as wheat, maize, rice and barley has included the modification of inflorescence architecture to improve grain yield and ease harvesting1. Yield increases have often been achieved through modifying the number and arrangement of spikelets, which are specialized reproductive branches that form part of the inflorescence. Multiple genes that control spikelet development have been identified in maize, rice and barley2–5. However, little is known about the genetic underpinnings of this process in wheat. Here, we describe a modified spikelet arrangement in wheat, termed paired spikelets. Combining comprehensive QTL and mutant analyses, we show that Photoperiod-1 (Ppd-1), a pseudo-response regulator gene that controls photoperiod-dependent floral induction, has a major inhibitory effect on paired spikelet formation by regulating the expression of FLOWERING LOCUS T (FT)6,7. These findings show that modulated expression of the two important flowering genes, Ppd-1 and FT, can be used to form a wheat inflorescence with a more elaborate arrangement and increased number of grain producing spikelets.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Doebley, J., Gaut, B. S. & Smith, B. D. The molecular genetics of crop domestication. Cell 127, 1309–1321 (2006).
Vollbrecht, E., Springer, P. S., Goh, L., Buckler, E. S. IV & Martienssen, R. Architecture of floral branch systems in maize and related grasses. Nature 436, 1119–1126 (2005).
Ashikari, M. et al. Cytokinin oxidase regulates rice grain production. Science 309, 741–745 (2005).
Miura, K. et al. OsSPL14 promotes panicle branching and higher grain productivity in rice. Nature Genet. 42, 545–549 (2010).
Ramsay, L. et al. INTERMEDIUM-C, a modifier of lateral spikelet fertility in barley, is an ortholog of the maize domestication gene TEOSINTE BRANCHED 1. Nature Genet. 43, 169–172 (2011).
Turner, A., Beales, J., Faure, S., Dunford, R. P. & Laurie, D. A. The pseudo-response regulator Ppd-H1 provides adaptation to photoperiod in barley. Science 310, 1031–1034 (2005).
Beales, J., Turner, A., Griffiths, S., Snape, J. W. & Laurie, D. A. A Pseudo-Response Regulator is misexpressed in the photoperiod insensitive Ppd-D1a mutant of wheat (Triticum aestivum L.). Theor. Appl. Genet. 115, 721–733 (2007).
Sharman, B. C. Branched heads in wheat and wheat hybrids. Nature 153, 497–498 (1944).
Sharman, B. C. Interpretation of the morphology of various naturally occurring abnormalities of the inflorescence of wheat (Triticum). Can. J. Bot. 45, 2073–2080 (1967).
Dobrovolskaya, O. et al. FRIZZY PANICLE drives supernumerary spikelets in bread wheat (T. aestivum L.). Plant Physiol. http://dx.doi.org/10.1104/pp.114.250043 (2014).
Yen, C. & Yang, J. L. The essential nature of organs in Gramineae, multiple secondary axes theory: A new concept. J. Sichuan Agric. Univ. 10, 544–565 (1992).
Koric, S . Branching genes in Triticum aestivum. Proc. Int. Wheat Genet. Symp. 4th, Colorado, Missouri, 283–288 (1973).
Pennell, A. L. & Halloran, G. M. Inheritance of supernumerary spikelets in wheat. Euphytica 32, 767–776 (1983).
Eagles, H. A., Cane, K. & Vallance, N. The flow of alleles of important photoperiod and vernalisation genes through Australian wheat. Crop Pasture Sci. 60, 646 (2009).
Huang, B. E. et al. A multiparent advanced generation inter-cross population for genetic analysis in wheat. Plant Biotech. J. 10, 826–839 (2012).
Wingen, L. U. et al. Molecular genetic basis of pod corn (Tunicate maize). Proc. Natl Acad. Sci. USA 109, 7115–7120 (2012).
Verbyla, A. P., Cullis, B. R. & Thompson, R. The analysis of QTL by simultaneous use of the full linkage map. Theor. Appl. Genet. 116, 95–111 (2007).
Pastina, M. M. et al. A mixed model QTL analysis for sugarcane multiple-harvest-location trial data. Theor. Appl. Genet. 124, 835–849 (2011).
Verbyla, A. P., Taylor, J. D. & Verbyla, K. L. RWGAIM: an efficient high-dimensional random whole genome average (QTL) interval mapping approach. Genet. Res. 94, 291–306 (2013).
Nakamichi, N., Kita, M., Ito, S., Yamashino, T. & Mizuno, T. PSEUDO-RESPONSE REGULATORS, PRR9, PRR7 and PRR5, together play essential roles close to the circadian clock of Arabidopsis thaliana. Plant Cell Physiol. 46, 686–698 (2005).
Li, C., Distelfeld, A., Comis, A. & Dubcovsky, J. Wheat flowering repressor VRN2 and promoter CO2 compete for interactions with NUCLEAR FACTOR-Y complexes. Plant J. 67, 763–773 (2011).
Díaz, A., Zikhali, M., Turner, A. S., Isaac, P. & Laurie, D. A. Copy number variation affecting the Photoperiod-B1 and Vernalization-A1 genes is associated with altered flowering time in wheat (Triticum aestivum). PLoS ONE 7, e33234 (2012).
Shaw, L. M., Turner, A. S. & Laurie, D. A. The impact of photoperiod insensitive Ppd-1a mutations on the photoperiod pathway across the three genomes of hexaploid wheat (Triticum aestivum). Plant J. 71, 71–84 (2012).
Shaw, L. M., Turner, A. S., Herry, L., Griffiths, S. & Laurie, D. A. Mutant alleles of Photoperiod-1 in wheat (Triticum aestivum L.) that confer a late flowering phenotype in long days. PLoS ONE 8, e79459 (2013).
Yan, L. et al. Positional cloning of the wheat vernalization gene VRN1. Proc. Natl Acad. Sci. USA 100, 6263–6268 (2003).
Zhao, T. et al. Characterization and expression of 42 MADS-box genes in wheat (Triticum aestivum L.). Mol. Genet. Genomics 276, 334–350 (2006).
Kobayashi, K. et al. Inflorescence meristem identity in rice is specified by overlapping functions of three AP1/FUL-like MADS box genes and PAP2, a SEPALLATA MADS box gene. Plant Cell 24, 1848–1859 (2012).
McSteen, P., Laudencia-Chingcuanco, D. & Colasanti, J. A floret by any other name: control of meristem identity in maize. Trends Plant Sci. 5, 61–66 (2000).
Endo-Higashi, N. & Izawa, T. Flowering time genes Heading Date 1 and Early Heading Date 1 together control panicle development in rice. Plant Cell Physiol. 52, 1083–1094 (2011).
Forster, B. P. et al. The barley phytomer. Ann. Bot. 100, 725–733 (2007).
Acknowledgements
We thank Bjorg Sherman for technical assistance with plant husbandry, Mark Talbot for expert assistance with scanning electron microscopy and Carl Davies for photography. We thank Lindsay Shaw and Megan Hemming for helpful discussions. A CSIRO O.C.E. Postdoctoral Fellowship funded S.A.B.
Author information
Authors and Affiliations
Contributions
S.A.B., C.C., K.R., J.G., E.J.F., B.T. and S.M.S. performed experiments and collected phenotypic information. C.C. and B.R.C. designed the MAGIC experiments, performed QTL and statistical analysis. S.A.B., E.J.F., B.T. and S.M.S. contributed new materials. All authors contributed to the preparation of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Rights and permissions
About this article
Cite this article
Boden, S., Cavanagh, C., Cullis, B. et al. Ppd-1 is a key regulator of inflorescence architecture and paired spikelet development in wheat. Nature Plants 1, 14016 (2015). https://doi.org/10.1038/nplants.2014.16
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/nplants.2014.16
This article is cited by
-
Chromosomal mapping of a major genetic locus from Agropyron cristatum chromosome 6P that influences grain number and spikelet number in wheat
Theoretical and Applied Genetics (2024)
-
Identification and functional analysis of a chromosome 2D fragment harboring TaFPF1 gene with the potential for yield improvement using a late heading wheat mutant
Theoretical and Applied Genetics (2024)
-
A high-resolution genotype–phenotype map identifies the TaSPL17 controlling grain number and size in wheat
Genome Biology (2023)
-
Identification and validation of quantitative trait loci for fertile spikelet number per spike and grain number per fertile spikelet in bread wheat (Triticum aestivum L.)
Theoretical and Applied Genetics (2023)
-
Mining novel genomic regions and candidate genes of heading and flowering dates in bread wheat by SNP- and haplotype-based GWAS
Molecular Breeding (2023)