Abstract
Background:
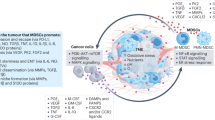
Myeloid-derived suppressor cells (MDSCs) are present in large numbers in blood of mice and humans with cancer, and they strongly inhibit T-cell and natural killer (NK) cell responses, at young and old age. We found that a highly attenuated bacterium Listeria monocytogenes (Listeriaat)-infected MDSC and altered the immune-suppressing function of MDSC.
Methods:
Young (3 months) and old (18 months) BALB/cByJ mice with metastatic breast cancer (4T1 model) were immunised with Listeriaat semi-therapeutically (once before and twice after tumour development), and analysed for growth of metastases and primary tumour, in relation to MDSC-, CD8 T-cell and NK cell responses.
Results:
We found that Listeriaat-infected MDSC, which delivered Listeriaat predominantly to the microenvironment of metastases and primary tumours, where they spread from MDSC into tumour cells (infected tumour cells will ultimately become a target for Listeria-activated immune cells). Immunotherapy with Listeriaat significantly reduced the population of MDSC in blood and primary tumours, and converted a remaining subpopulation of MDSC into an immune-stimulating phenotype producing IL-12, in correlation with significantly improved T-cell and NK cell responses to Listeriaat at both ages. This was accompanied with a dramatic reduction in the number of metastases and tumour growth at young and old age.
Conclusions:
Although preclinical studies show that immunotherapy is less effective at old than at young age, our study demonstrates that Listeriaat-based immunotherapy can be equally effective against metastatic breast cancer at both young and old age by targeting MDSC.
Similar content being viewed by others
Main
With the current rise of the elderly population, cancer will continue to remain an increasingly frequent disease and a major cause of death. From 2010 to 2030, the total projected cancer incidence in the United States for older adults will increase by approximately 67% (Smith et al, 2009). In particular, metastatic cancer has already surpassed heart disease as the primary cause of mortality in adults younger than the age of 85 years (Jemal et al, 2005). In spite of some improvements in prevention and treatment, metastatic cancer is still the most common cause of death in the elderly, with comorbid conditions complicating further treatment. When cancer becomes metastatic, it often needs aggressive second-line treatment, for which the options are few. This is particularly challenging for frail, elderly cancer patients in which comorbidity has an antagonistic role (Extermann, 2011). Immunotherapy may be the best and most benign option for preventing or curing metastatic cancer in such patients. Unfortunately, cancer immunotherapy is less effective at old than at young age, due to T-cell unresponsiveness, especially in the tumour microenvironment (TME; Provinciali et al, 2003; Lustgarten et al, 2004; Castro et al, 2009; Gravekamp, 2011). Various causes have been described for T-cell unresponsiveness at old age, such as lack of naive T cells at older age, deficiency in the upregulation of co-stimulatory molecules on aged dendritic cells (DCs), and most recently, the increase in the population of MDSC in the TME of old compared with young mice, among other age-related immune impairments (Utsuyama et al, 1992; George and Ritter, 1996; Miller, 1996; Grizzle et al, 2007; Gravekamp, 2011; Lefebvre et al, 2012; Weinberger and Grubeck-Loebenstein, 2012).
Myeloid-derived suppressor cells are a heterogeneous population of myeloid progenitor cells, that is immature granulocytes, macrophages, and DCs (Gabrilovich and Nagaraj, 2009). In healthy individuals, the immature myeloid cells differentiate into mature granulocytes, macrophages, and DC. In cancer patients, MDSCs migrate to the primary tumour, which blocks their differentiation and activates these immature cells to produce immune suppressive factors, such as arginase I, inducible nitric oxide synthetase, and reactive oxygen species (ROS), and also cytokines such as IL-6, IL-10, and TGF-β, which are able to downregulate antigen-specific and nonspecific T-cell responses in the TME (Gabrilovich and Nagaraj, 2009; Ostrand-Rosenberg and Sinha, 2009). In mice, MDSC express both the myeloid lineage differentiation antigen Gr1 (Ly6C and Ly6G) and the αM integrin CD11b. Two major groups of MDSC have been described: CD11b+Gr1high (CD11b+Ly6G+Ly6Clow) with a granulocytic MDSC (gMDSC) phenotype, and CD11b+Gr1low (CD11b+Ly6G−Ly6Chigh) with a monocytic MDSC (mMDSC) phenotype (Movahedi et al, 2008; Youn et al, 2008). Both mMDSC and gMDSC are immunosuppressive but may have different functions at the tumour site.
Although Listeriaat originally has been used to deliver tumour-associated antigens (TAAs) into antigen-presenting cells for the activation of T cells against their own tumours (Pan et al, 1999; Singh et al, 2005; Seavey et al, 2009), we discovered recently that the Listeriaat bacteria also infects tumour cells and then kills the tumour cells directly through the production of high levels of ROS, and that Listeriaat-activated CD8 T cells significantly reduces tumour growth at young age, as shown by in vivo depletion of CD8 T cells in Listeriaat-immunised tumour-bearing mice (Kim et al, 2009). In the study presented here, we discovered an additional novel pathway of Listeriaat. We found an intimate relationship between Listeriaat and MDSC that is particularly interesting for tailoring immunotherapy to elderly cancer patients, as the number of MDSC increases with age in the TME, and MDSC contributes to the age-related T-cell unresponsiveness (Grizzle et al, 2007). This unique relationship resulted in the selective delivery of Listeriaat, with the help of MDSC, to the metastases and primary tumour, where it infected and killed tumour cells without having side effects in normal tissues. In addition, Listeriaat altered a subpopulation of MDSC into an immune-stimulating phenotype in correlation with improved CD8 T-cell and NK cell responses to Listeriaat, and a dramatic decrease in the number of metastases and tumour growth at young and old age. The results of this study strongly suggest that MDSC are an imperative target for immunotherapy at both young and old age.
Materials and Methods
Mice
Normal female BALB/cByJ mice (3 months and 18 months) were obtained from NIA/Charles River and maintained in the animal husbandry facility at Albert Einstein College of Medicine according to the Association and Accreditation of Laboratory Animal Care guidelines. All mice were kept under Bsl-2 conditions as required for Listeriaat immunisations.
Cells and cell culture
The 4T1 cell line, derived from a spontaneous mammary carcinoma in a BALB/c mouse and is highly metastatic in a BALB/cByJ background (kindly provided by Dr F Miller, University of Michigan, Ann Arbor; Aslakson and Miller, 1992). 4T1 cells were cultured in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovine serum (FBS), 1 mM mixed nonessential amino acids, 2 mM L-glutamine, insulin (0.5 USP units per ml), penicillin (100 U per ml), and streptomycin (100 μg ml−1; Pen/Strep).
Attenuated Listeriaat
A highly attenuated Listeria monocytogenes (Listeriaat) has been used for immunisations of the 4T1 model, as previously described (Kim et al, 2008). The Listeria plasmid, pGG-34, expresses the positive regulatory factor (prfA) and Listeriolysin O (LLO; Gunn et al, 2001). prfA regulates the expression of other virulence genes, and is required for survival in vivo and in vitro. The background strain XFL-7 lacks the prfA gene, and retains the plasmid in vitro and in vivo (Gunn et al, 2001). The coding region for the C-terminal part of the LLO (cytolytic domain that binds cholesterol in the membranes) protein in the plasmid has been deleted, but Listeriaat is still able to escape host vacuole (Singh et al, 2005). Mutations have been introduced into the prfA gene and the remaining LLO (expressed by the pGG34 vector), which further reduced the pathogenicity of Listeria (Singh et al, 2005).
Semi-therapeutic immunisation protocol and tumour challenge
BALB/cByJ mice were immunised with Listeriaat and challenged with 4T1 tumour cells as previously described (Kim et al, 2009). Briefly, one preventive immunisation with 0.5 × 107 CFU of Listeria (LD50=108 CFU) per 500 μl saline, or saline alone was administered intraperitoneally (i.p.) before tumour challenge at day 0, followed by tumour challenge at day 4 (0.5 × 105 4T1) in the mammary fat pad, followed by two additional therapeutic immunisations i.p. with 0.5 × 107 CFU of Listeria per 500 μl saline, or saline alone at days 7 and 14. The mice were euthanised 2 days after the last immunisation, and analysed for immune responses, and for tumour weight, frequency, and location of metastases. All untreated 4T1 mice developed a primary tumour that extended to the chest cavity lining and metastasised predominantly to the mesenteric lymph nodes, and less frequently to the diaphragm, portal liver, spleen, and kidneys within 14 days (metastases were visible as nodules and counted by eye) as described previously (Kim et al, 2008).
ELISPOT and ELISA
Spleen cells were isolated from Listeriaat-immunised and control mice (2 days after the last immunisation) and analysed for NK cell and T-cell responses by ELISPOT as described previously (Kim et al, 2008). To detect Listeriaat-induced immune responses, 2 × 105 isolated spleen cells were infected with 2 × 105 CFU of Listeriaat for 1 h, and subsequently treated with gentamicin (50 μg ml−1) to kill all extracellular bacteria but not the intracellular bacteria until the end of re-stimulation (72 h). After the 72 h, the frequency of IFN-γ-producing cells was determined by ELISPOT according to standard protocols (BD Pharmingen, San Diego, CA, USA) using an ELISPOT reader (CTL Immunospot S4 analyzer, Cellular Technology Ltd, Cleveland, OH, USA). To determine NK and CD8 T-cell responses, spleen cells were depleted for NK (CD49b) or CD8 T cells using magnetic bead depletion techniques according to the manufacturer’s instructions (Miltenyi Biotec, Auburn, CA, USA). All antibodies were purchased from BD Pharmingen.
The production of IL-12 by gMDSC and mMDSC was also measured by ELISA. For this purpose, 500 000 gMDSC or mMDSC were incubated with Listeriaat in 200 μl RPMI medium containing 10% FBS at various ratios, as indicated in the text, for 1 h, then cultured in gentamicin (50 μg ml−1) for 72 h. At the end of the 72 h, levels of IL-12p70 were determined in the culture supernatant by ELISA according to manufacturer’s instructions (BD Pharmingen).
Isolation and analysis of MDSC
Granulocytic MDSC and mMDSC were isolated from the spleens of 4T1 tumour-bearing mice, according to the manufacturer’s instructions (Myeloid-Derived Suppressor Cell Isolation Kit, Miltenyi Biotec). Briefly, the gMDSC (Ly6G+) were positively selected using anti-Ly6G-biotin and anti-biotin microbeads. In a second purification step, a mMDSC-enriched fraction (Gr1dimLy6G−) was obtained from the flow-through population (Ly6G−), by positive sorting with anti-Gr1-biotin and streptavidin microbeads. For separation of the magnetically labelled cells, the Automacs Proseparator (Miltenyi Biotec) was used. As determined by flow cytometry, the purity of the isolated gMDSC was ⩾90% and mMDSC ⩾85%.
Flow cytometry analysis
Immune cells from spleen, blood, and primary tumours from individual mice were isolated as described previously (Kim et al, 2008; Castro et al, 2009) Briefly, red blood cells from blood or tumour cells were lysed according to standard protocols, and the remaining leukocyte population was used for analysis. Single-cell suspensions were obtained from primary tumours using GentleMacs combined with a mild treatment of the cells using collagenase, dispase, and DNAse I, according to the manufacturers instructions (Miltenyi Biotec).
Cells were first incubated with an Fc blocked (anti-CD16), and subsequently with the antibodies for the identification of different cell types. For MDSC, anti-CD11b and -Gr1, antibodies were used. The CD11b+Gr1high population represents the gMDSC population and the CD11b+Gr1low the mMDSC population. To detect the production of intracellular cytokines, the cytofix/cytoperm kit from BD Pharmingen was used according to manufacturer’s instructions, and antibodies to IL-12 was used. Appropriate isotype controls were used for each sample. Depending on the sample size, 10 000–50 000 cells were acquired by scanning using a Fluorescence-Activated Cell Sorter (flow cytometry; Beckton and Dickinson, Franklin Lakes, NJ, USA; Excalibur), and analysed using FlowJo 7.6 software (FACS caliber, Ashland, OR, USA). Cell debris and dead cells were excluded from the analysis based on scatter signals and use of Fixable Blue or Green Live/Dead Cell Stain Kit (Invitrogen, Grand Island, NY, USA). All antibodies were purchased from BD Pharmingen or eBiosciences, (San Diego, CA, USA).
Isolation of Listeriaat from tumours, metastases, and normal tissue
Mice with 4T1 metastases and tumours were immunised once with Listeriaat (0.5 × 107 CFU), and euthanised at various time points. Metastases, tumours, and spleens were dissected and treated with gentamicin for 1 h, and then washed to remove the dead bacteria and gentamicin. Subsequently, metastases, tumours, and spleens were homogenised, plated on agar, and counted for Listeriaat colonies the next day. The number of Listeriaat was calculated per gram tissue.
Infection of gMDSC and mMDSC with Listeriaat in vitro
Granulocytic MDSC and mMDSC were isolated from spleens of mice with large 4T1 tumours (1 cm in diameter) using the Miltenyi kit as described above, and infected with Listeriaat in a 1 : 10 ratio, then cultured for 1 h in RPMI containing 10% FBS. Subsequently, the infected cells were treated with gentamicin (50 μg ml−1) for 1 h, and washed to remove the dead bacteria and gentamicin. Subsequently, the infected cells were cultured and stopped at various time points. To measure the number of live Listeriaat bacteria, the infected cells were lysed in water, plated on agar, and counted for Listeriaat colonies the next day. The number of Listeriaat CFU was calculated per 106 cells.
Confocal microscopy
Tissues from mice were snap-frozen in optimal cutting medium (TissueTek; Sakura, Torrance, CA, USA). Ten-micrometer sections were cut using a cryotome and mounted on slides. Sections were fixed with 3.7% formaldehyde for 10 min at room temperature (RT). 4T1 tumour cells and MDSCs were cultured with or without Listeriaat as described in the text and fixed in 3.7% formaldehyde. Cells were first permealised with 0.1% Triton X-100 in PBS for 5 min, followed by blocking (1% normal goat serum) and washing steps, and then incubated with the primary Abs for Listeria (IgG1 isotype, Difco Listeria O antiserum Poly serotype 1,4; BD (Grand Island, NY, USA), cat. #223021) in PBS with 0.03% BSA, 0.1% Triton X-100, for 90 min at RT, followed by washing steps, and then incubated with the secondary antibody goat anti-rabbit IgG-Cy3-labelled (dilution 1 : 500; Abcam, Cambridge, MA, USA) for 60 min at RT, followed by incubation with Alexa Fluor 488 Phallodin (40 U ml−1; LifeTechnologies, Grand Island, NY, USA) for 20 min at RT to stain the cytoplasm. Slides were mounted with DAPI containing mounting medium (Vectashield; Vector Laboratories, Burlingham, CA, USA), and analysed on a Leica SP2 confocal microscope with a × 63 oil immersion objective equipped with AOBS system (Leica, Buffalo, NY, USA), using Image J software.
Statistical analysis
To statistically analyse the effects of Listeriaat on the growth of metastases and tumours and immune responses in the 4T1 model, the unpaired t-test and the analysis of variance (one-way) were used. Values P<0.05 were considered statistically significant. *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001 are significant.
Results
Behaviour of Listeriaat in the TME, normal tissues, and MDSC
We analysed the behaviour of Listeriaat in BALB/cByJ mice with metastatic breast cancer (4T1 model). Tumour-bearing mice were injected once with 0.5 × 107 Listeriaat bacteria and analysed for the number of CFU of Listeriaat in tumours, metastases (predominantly in the mesenteric lymph nodes, and less frequently in the diaphragm, portal liver, spleen, and kidneys), and normal tissue such as the spleen, at various time intervals (days 1, 3, and 4). We found that Listeriaat multiplied and survived in metastases (of all locations) and primary tumours but not in normal tissues in vivo (Figure 1A). In addition, we analysed the CFU of Listeriaat in other normal tissues, such as lung, liver, heart, gastrointestinal tract, kidneys, spleen, and blood, of mice with and without 4T1 tumours at various time intervals (days 1, 3, and 7). We found again that Listeriaat did not multiply in normal tissues, with an exception of liver and spleen in mice with tumours because these organs were already metastasised (see Supplementary Figure 1). However, in vitro Listeriaat efficiently infected and killed primary cultures of normal murine and human epithelial cells (for more detail see Supplementary Information). The reason for this discrepancy in survival of Listeriaat in normal cells between the in vivo and in vitro situation is the presence of an intact immune system in normal tissues in vivo that is absent in vitro. Moreover, TME is highly immune suppressive and protects Listeriaat from immune clearance, whereas in normal tissues that lacks immune suppression Listeriaat is rapidly cleared by macrophages, NK cells, and T cells (Muraille et al, 2007; Zenewicz and Shen, 2007).
Survival and multiplication of Listeriaat in the TME, normal tissues, and MDSC.(A) Listeriaat multiplied in metastases and primary tumour but not in spleen (tumour-free tissue) in vivo. 4T1 tumour-bearing mice were injected once with 0.5 × 107 Listeriaat and analysed for the number of Listeriaat in primary tumour, metastases, and spleen at different time intervals. n=3 mice per group. Mice were individually analysed and the results were averaged. The graph is a representative of three experiments. (B) Listeriaat infected both types of MDSC but multiplies in the mMDSC population in vitro. Splenic MDSC of tumour-bearing mice were isolated and cultured with Listeriaat at a 1 : 10 ratio for an hour, and then gentamicin was added, and terminated at different time points. The infected cells were lysed in water and plated onto LB agar to determine the number of Listeriaat CFU the next day. n=3 mice per group. Results of two experiments were averaged. Analysis of variance (ANOVA, one-way) *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001 is significant. (C) The number of Listeriaat CFU in tumour MDSC was much higher than in splenic MDSC. Tumour-bearing mice were injected with Listeriaat as in A. The next day, mice were euthanised and MDSC were isolated from tumours and spleens, and analysed the MDSCs for their number of Listeriaat CFU. n=3 mice. Mice were individually analysed and the results were averaged, representative of two experiments. Unpaired t-test P<0.05 is statistically significant. (D) mMDSC delivered Listeriaat predominantly to the tumour microenvironment. mMDSC and gMDSC isolated from spleens of tumour-bearing mice were infected in vitro with Listeriaat, and injected (107 cells) into the tail vein of tumour-bearing mice. Next day, the number of Listeriaat CFU was determined in the primary tumours, metastases, and spleens. Mice were individually analysed and the results were averaged, representative of two experiments. n=5 mice per group ANOVA (one-way) *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001 is significant. The error bars on all graphs represent the s.e.m. (E) Listeriaat spread from MDSC to tumour cells. Isolated splenic MDSC from tumour-bearing mice were infected with Listeriaat for 1 h, treated with gentamicin, and then cultured with 4T1 tumour cells (that has never been exposed to Listeriaat before) in vitro in the presence of gentamicin, and the cultures were finally terminated at various time points. Left: Listeriaat migrating from MDSC into tumour cell (after 6 h). Middle: Listeriaat in cytoplasm of dying tumour cell (after 12 h). Right: Listeriaat in cytoplasm of dying tumour cell (after 24 h). Listeriaat are red (Cy-3) and nuclei blue (DAPI), and cytoplasm green (actin staining). (F) Listeriaat-infected MDSC at young and old age. Splenic MDSC were isolated from young and old tumour-bearing mice and infected with Listeriaat as described in E. Left: Listeriaat in the cytoplasm of MDSCs from young mice. Right: Listeriaat in the cytoplasm of MDSC from old mice.
It is known that MDSC control immune suppression in the TME in human and mice, and that they are attracted to the TME through the production of chemo-attractants including granulocyte–macrophage colony-stimulating factor, which is highly produced by the 4T1 tumour cells (Gabrilovich and Nagaraj, 2009; Ostrand-Rosenberg and Sinha, 2009; Pylayeva-Gupta et al, 2012). Therefore, we next analysed the interaction between Listeriaat and MDSC in vitro and in vivo. To analyse the relationship between Listeriaat and MDSC in vitro, the gMDSC and mMDSC populations were isolated from the spleens of young tumour-bearing mice. We determined whether Listeriaat could infect and multiply in both types of MDSC. For this purpose, both gMDSC and mMDSC were cultured with Listeriaat at a 1 : 10 ratio for 1 h, then treated with gentamicin (to clear all extracellular bacteria), and terminated at different time points. The infected cells were lysed in water and plated on agar to determine the number of Listeriaat CFU the next day. It appeared that the Listeriaat infected both types of MDSC but multiplied in the mMDSC and not in the gMDSC in vitro (Figure 1B). Granulocytic MDSC and mMDSC in the spleens of mice without tumours (3%–5%) were analysed as well, but Listeriaat did not multiply in the MDSC of these non-tumour-bearing mice (see Supplementary Figure 2).
We also analysed the relationship between Listeriaat in gMDSC and mMDSC in vivo. For this purpose, tumour-bearing mice were immunised once i.p. with 0.5 × 107 Listeriaat CFU. The next day, these mice were euthanised, and mMDSC and gMDSC were isolated from their tumours and spleens, then treated with gentamicin, lysed in water, and plated on agar to determine the CFU of Listeriaat. The number of Listeriaat CFU in mMDSC was approximately twice as high as in gMDSC for both primary tumours and spleen (Figure 1C). However, the number of CFU isolated in the primary tumours was ∼63-fold higher than isolated from the spleens. These results demonstrate again that Listeriaat persists and replicates in the TME but not in normal tissues like the spleen. To prove that mMDSC delivers Listeriaat predominantly to the TME in vivo, we isolated gMDSC and mMDSC from the spleens of tumour-bearing mice, infected them with Listeriaat for 1 h, treated with gentamicin, and then injected the infected gMDSC and mMDSC into the tail vein of tumour-bearing mice as two separate groups. Next day we isolated CFU of Listeriaat from tumours, metastases, and spleen. Listeriaat was predominantly isolated from primary tumours and metastases of mice that received infected mMDSC (Figure 1D).
We then hypothesised that if MDSC predominantly deliver Listeriaat to the metastases and tumours, then we expect that Listeriaat could spread from MDSC into tumour cells through a cell-to-cell spread mechanism characteristic of Listeria (Portnoy et al, 2002). To prove this hypothesis, we infected MDSCs in vitro with Listeriaat, treated them with gentamicin, then cultured the infected MDSC with the 4T1 tumour cells (that had never been exposed to Listeriaat before) in vitro in the presence of gentamicin, and finally terminated the cell cultures at various time points. Many Listeriaat bacteria were found inside tumour cells after 12 and 24 h, indicating that Listeriaat spreads from MDSC into tumour cells (Figure 1E). Finally, we confirmed that Listeriaat infects MDSC not only at young but also at old age (Figure 1F).
Listeriaat significantly reduced the percentage of MDSC in blood and primary tumours at both young and old age
After demonstrating that Listeriaat can infect MDSC at young and old age, we analysed whether Listeriaat could alter the number and function of MDSC in blood and primary tumours of the 4T1 model. Myeloid-derived suppressor cells are present in large numbers in blood of humans and mice with cancer (Diaz-Montero et al, 2009; Ilkovitch and Lopez, 2009), and strongly inhibit T-cell responses in the TME at young and old age (Gabrilovich and Nagaraj, 2009; Ostrand-Rosenberg and Sinha, 2009). Here, we studied the semi-therapeutic effects of three Listeriaat immunisations (one before and two after tumour development) on MDSC in blood and tumours of the 4T1 model at young (3 months) and old (18 months) age. This immunisation protocol was chosen because in an earlier study we have shown that CD8 T cells could be stimulated by Listeriaat in spleens of 4T1 tumour-bearing mice at young age (Kim et al, 2009). In the study presented here, we analysed whether Listeriaat could also activate CD8 T cells at old age in 4T1 tumour-bearing mice. As CD8 T-cell responses are controlled by MDSC, we first analysed the effect of Listeriaat on MDSC at young and old age.
In total blood of tumour-bearing mice, the percentage of MDSC was extremely high at young (∼81%) and old (∼68%) age (Figure 2). These levels were strongly reduced by Listeriaat immunisations compared with the non-treated (saline) mice at both ages. The MDSC population in total blood was dominated by gMDSC at both ages (Figure 2). Only a few percent of the total blood population represented mMDSC, and relatively little effect of Listeriaat on these mMDSC was observed.
Listeriaat immunisations significantly reduced the percentage of gMDSC in blood of both young and old mice.Mice were immunised with Listeriaat on days 0, 7, and 14, and challenged with 4T1 tumour cells on day 4. All mice were euthanised on day 16 and analysed by flow cytometry for the total MDSC population (CD11b+Gr1+), the gMDSC population (CD11b+Gr1high), and the mMDSC population (CD11b+Gr1low), in blood of young and old mice. All MDSCs were gated within the total live leukocyte population of the blood. Graphs are the average of three experiments (mice were individually analysed). n=5 mice per group. Unpaired t-test. *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001 is significant. The error bars represent the s.e.m. A representative example of gating MDSC of young and old mice is shown in Supplementary Figure 6 of the Supplementary Information. In addition, a representative example of absolute numbers of MDSC, gMDSC, and mMDSC in blood of young and old mice is shown in Supplementary Figure 7 of the Supplementary Information.
In the primary tumours (CD45+ population), the percentage of MDSC was considerably lower compared with blood at both ages. However, the percentage of MDSC in the primary tumours at old age was two to three times higher than at young age. Such phenomenon was observed earlier (Grizzle et al, 2007). Listeriaat immunisations significantly reduced the percentage of MDSC in the primary tumours at both ages, but most dramatically in old mice. In the primary tumour, the MDSC population was dominated by gMDSC at both ages, but this was more visible at old than at young age (Figure 3). The Listeriaat immunisations significantly reduced the percentage of gMDSC at both ages. However, the net effect was more robust at old than at young age. The effect of Listeriaat on mMDSC, although significant, was much less abundant compared with the effect of Listeriaat on gMDSC (Figure 3).
Listeriaat immunisations significantly reduced the percentage of gMDSC in primary tumours of both young and old mice.All mice were immunised with Listeriaat, challenged with 4T1 tumour cells, and euthanised as described in Figure 2. The total MDSC population (CD11b+Gr1+), as well as the gMDSC population (CD11b+Gr1high) and the mMDSC population (CD11b+Gr1low), were gated within the total live CD45+ population of the tumour cell suspension, and analysed by flow cytometry. Graphs are the average of three experiments (mice were individually analysed). n=5 mice per group. Unpaired t-test. *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001 is significant. The error bars represent the s.e.m. A representative example of gating MDSC of young and old mice is shown in Supplementary Figure 6 of the Supplementary Information.
Listeriaat significantly increased the production of IL-12 by mMDSC in blood at both young and old age
IL-12 is an important activator of naive and mature T cells (Hsieh et al, 1993; Valenzuela et al, 2002). It has been reported that Listeriaat induces the production of IL-12 in macrophages (Kim et al, 2003). We found that semi-therapeutic immunisations with Listeriaat significantly improved the intracellular production of IL-12 by mMDSC in vivo in blood of young and old 4T1 tumour-bearing mice, and less vigorous but still significant in gMDSC (Figure 4A). In addition, IL-12 production by mMDSC and gMDSC was also analysed in vitro. For this purpose, gMDSC and mMDSC were isolated from spleen of tumour-bearing mice at young and old age, and infected with Listeriaat. We found that the production of IL-12 in the culture supernatant of gMDSC and mMDSC infected with Listeriaat was increased at young and old age (Figure 4B). Again, we found that the IL-12 production was much more vigorous by mMDSC than gMDSC. The small primary tumours of Listeriaat-treated mice at young and old age exhibited too low number of IL-12-producing MDSC, and reliable analysis was not possible.
Listeriaat immunisations significantly increased the production of IL-12 by MDSC in blood of both young and old mice.All mice were immunised with Listeriaat, challenged with 4T1 tumour cells, and euthanised as described in Figure 2. The gMDSC and mMDSC were gated in the blood as shown in Supplementary Figure 6 of the Supplementary Information, and analysed for the intracellular production of IL-12 by flow cytometry (A). n=3–5 mice per group. Mice were individually analysed and the results of three experiments were averaged. Unpaired t-test. *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001 is significant. The error bars represent the s.e.m. The IL-12 production by gMDSC and mMDSC was also analysed in vitro. For this purpose, gMDSC and mMDSC were isolated from spleens of tumour-bearing mice as described in Figure 1, infected with Listeriaat at various ratio’s (gMDSC or mMDSC/Listeriaat=1:0, 0.01, and 0.1) for 1 h, then cultured for 72 h in the presence of gentamicin, and then analysed for the production of IL-12 in the culture supernatant by ELISA (B). This experiment was performed two times and the results were averaged. Unpaired t-test P<0.5 is statistically significant. The error bars represent the s.e.m.
Listeriaat improved T-cell and NK cell responses at young and old age
If Listeriaat reduces the percentage of MDSC (directly or indirectly) and improves the production of IL-12 in both age groups, then improvement of T-cell and NK cell responses could be expected as well. The production of IFN-γ was used as activation marker for both CD8 T cells and NK cells (Listeriaat does not induce the production of IL-2; Kim et al, 2008). We found that the number of IFN-γ-producing cells in the spleen of mice that received Listeriaat was significantly higher than in the control group (saline), upon re-stimulation with Listeriaat, although the response was more abundantly at young (Figure 5A) than at old age (Figure 5B). In addition, depletion of the CD8 T cells or NK cells from the spleen cell population resulted in a decrease in the number of IFN-γ-producing cells. Therefore, we concluded that both CD8 T cells and NK cells were responsible for the increase in IFN-γ-producing cells upon re-stimulation with Listeriaat (Figure 5A and B).
Listeriaat-activated CD8 T cells and NK cells at both young and old age.All mice were immunised with Listeriaat, challenged with 4T1 tumour cells, and euthanised as described in Figure 2. Splenocytes were isolated from tumour-bearing young and old mice, pooled in each group, re-stimulated with Listeriaat, and analysed for the number of IFN-γ-producing CD8 T cells and NK cells by ELISPOT. CD8 T cells and NK cells were depleted by magnetic bead technique. The number of IFN-γ-producing spots was determined per 200 000 splenocytes. n=5 mice per group. This experiment was performed two times and the results were averaged. Unpaired t-test. *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001 is significant. The error bars represent the s.e.m.
Listeriaat-based immunotherapy was equally effective at young and old age
Based on the equal results with Listeriaat at young and old age, that is, reduction in the percentage of MDSC, increase in the production of IL-12, and improvement of CD8 T-cell and NK cell responses to Listeriaat, we expect that Listeriaat-based immunotherapy should be effective at both ages. The semi-therapeutic immunisations with Listeriaat almost completely eliminated all metastases (Figure 6A), and significantly reduced tumour growth at both ages (Figure 6B).
Listeriaat immunisations significantly reduced the number of metastases and tumour growth in both young and old mice.All mice were immunised with Listeriaat, challenged with 4T1 tumour cells, and euthanised as described in Figure 2. The number of metastases (A) and tumour weight (B) was determined. n=5 mice per group. This experiment was performed three times and the results were averaged. Unpaired t-test. *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001 is significant. The error bars represent the s.e.m.
Discussion
Various groups have analysed cancer vaccination at young and old age in preclinical animal models. The group of Lustgarten showed that young but not old mice developed long-lasting memory responses to a pre-B-cell lymphoma (BM-185). They found that adding anti-OX40 or anti-4-1BB mAb to a DC-based vaccine, resulted in vigorous anti-tumour responses in a syngeneic TRAMP-C2 model at young and old age, whereas without anti-OX40 or anti-4-1BB, protection was significantly better in young than in old mice (Sharma et al, 2006). Moreover, immunisation of young and old mice with DC-TRAMP-C2 vaccine plus anti-OX40 or anti-4-1BB mAb resulted in improved CD8 T-cell responses to apoptotic TRAMP-C2 cells in vitro upon re-stimulation, compared with the same vaccination without OX40 or anti-4-1BB mAb at old age, but the CD8 T responses were still less vigorous compared with the same immunisations at young age. The group of Provinciali demonstrated that just adding IL-2 to a tumour cell-based vaccine improved anti-tumour responses but not memory responses to the tumour at old age (Provinciali et al, 2000). However, in a later study they found that the efficacy of DNA vaccination against cancer at old age could be improved through electroporation of the plasmid DNA (Provinciali et al, 2012). Our group showed that CD8 T cells could be significantly improved in tumour-bearing mice to TAA Mage-b by DNA vaccination at young but not at old age (Castro et al, 2009). Despite these studies, most, if not all, cancer vaccines tested in human clinical trials have been pre-clinically tested in young subjects only. Lack of tailoring cancer vaccination to older age may have been partly responsible for the moderate success in cancer patients whom the majority are aged 65 years and over. The study presented here provides evidence that cancer immunotherapy can be improved at old age by targeting various cell types of the innate and adaptive immune system.
We demonstrated that Listeriaat-based immunotherapy was successful against metastatic breast cancer at both young and old age, and that an intimate relationship between Listeriaat and MDSC significantly contributed to this success. We found that (1) Listeriaat-infected MDSC, (2) MDSC delivered Listeriaat predominantly to the TME (primary tumour and metastases), and (3) Listeriaat spread from MDSC into tumour cells. In previous studies, we have shown that Listeraat-induced ROS kills tumour cells directly (Kim et al, 2009). Our results strongly suggest that the infected MDSC contributed to activation of T cells and NK cells. For instance, we found that the percentage of MDSC in tumour-bearing mice significantly reduced in blood at young and old age by semi-therapeutic immunisations with Listeriaat, which may have contributed to lower immune suppression. In addition, we found that Listeriaat altered a subpopulation of immune suppressive MDSC into an immune-stimulating phenotype, producing high levels of IL-12 in blood of 4T1 tumour-bearing mice at young and old age. Also in humans with cancer, MDSC are present in large numbers in blood (Diaz-Montero et al, 2009), although their phenotype is somewhat different from mice. In humans, CD11b+CD33+CD14+CD15+HLA-DR− represents MDSC. We infected human MDSC with Listeriaat in vitro and found similar results, that is, a significant increase in the population of IL-12-producing MDSC compared with non-infected MDSC (see Supplementary Figure 3). These results suggest that Listeriaat-based vaccine platforms may have potential to improve the efficacy of cancer immunotherapy.
IL-12 is known for activating naive and mature T cells (Hsieh et al, 1993; Valenzuela et al, 2002) and may have contributed to the CD8+ T cell activation by Listeriaat in spleens of tumour-bearing mice at young and old age. Although the number of CD8+ T cells producing IFN-γ was lower at old than at young age (unpaired t-test P=0.0004), it was still high and significantly different compared with the saline control group (unpaired test P=0.0003). The group of Nikolich-Zugich also reported lower T-cell responses (proliferation) to Listeria at old than at young age, and they found that this was due to age-related alterations in CD8α DC (Li et al, 2012), a phenomenon that may have happened in our study as well. In a previous study, we have shown in young mice receiving semi-therapeutic immunisations with Listeriaat that depletion of CD8 T cells in vivo resulted in re-growth of primary tumours by 50% compared with all control groups (saline or isotype control; Kim et al, 2009), indicating that Listeriaat-activated T cells contributed to tumour cell destruction in vivo. The new concept here is that Listeriaat also infects tumour cells in vivo, thereby changing the tumour cells into a sensitive target for Listeriaat-activated T cells because infected tumour cells express Listeriaat proteins (Kim et al, 2009). As Listeriaat-activated CD8+ T cells at young and old age, it is expected that they have killed Listeriaat-infected tumour cells at both ages. Not only CD8 T cells but also NK cells were strongly activated by Listeriaat in the spleens of young and old mice, and may have contributed to the destruction of infected tumour cells as well. Listeriaat is not only effective against metastatic breast cancer but also against metastases in a pancreatic cancer model Panc-02, although this has been tested at young age only so far (see Supplementary Figure S4).
A question raised during this study was how the Listeriaat bacteria could reduce the population of MDSC so efficiently. It is possible that the Listeriaat destroyed the gMDSC directly like they kill tumour cells, that is, through the production of high levels of ROS. Alternatively, it is possible that Listeriaat efficiently killed tumour cells in the early phase of treatment and prevented the MDSC migrating from the bone marrow to the primary tumour and metastases. It is also possible that Listeriaat-infected gMDSC become a target for Listeriaat-activated CD8 T cells (or NK cells) because of the IL-12 production in MDSC. Others have shown that CD8 T cells can eliminate MDSC through Fas–FasL interaction (Sinha et al, 2011), which may have happened here as well. In conclusion, various pathways may lead to the reduction in MDSC and more analysis is required.
We also tested whether Listeriaat-based immunotherapy could be valuable for clinical application. Initially, the results were disappointing. Little effect was observed after three therapeutic immunisations with the high dose of Listeriaat (0.5 × 107 CFU) once a week. However, the Listeriaat is eliminated by the immune system in normal tissues within 3–5 days, indicating that Listeriaat could infect MDSC and tumour cells only in the first few days. To establish a continuous presence of Listeriaat that can infect MDSCs and tumour cells without inducing listeriosis, we tested various doses of Listeriaat administered with various frequencies and time intervals in the 4T1 model. It appeared that 104 CFU of Listeriaat every other day was much more effective against the metastases than 0.5 × 107 once a week. Using this optimised protocol, we found that five therapeutic immunisations with 104 CFU of Listeriaat reduced the number of metastases almost as good as after the semi-therapeutic immunisations both at young and old age, but little effect was observed on the primary tumours (see Supplementary Figure 5). The dose of Listeriaat may have been too low to kill all tumour cells in the primary tumours or to sufficiently reduce immune suppression in the TME. Therefore, addition of agents that further reduces immune suppression or may eliminate the primary tumour may lead to the complete elimination of metastatic breast cancer at young and old age. Currently, we have various combination therapies under investigation, such as Listeriaat and curcumin, an agent that reduces IL-6 or cyclic di-guanylate, an intracellular signaling molecule that activates T cells, to further improve cancer vaccination at older age. In addition, inclusion of TAAs such as Mage-b into the Listeriaat (Mage-b is expressed by the 4T1 tumour and is homologous with human MAGE; De Backer et al, 1995), may even further improve the vaccine efficacy of the Listeriaat-based cancer immunotherapy against the metastases and primary tumours. Therapeutic immunisation protocols with Listeriaat expressing TAA Mage-b are currently being tested in the 4T1 model.
The Listeriaat of this study is highly attenuated and therefore non-pathogenic and non-toxic, even at old age. Extensive pathology studies of mice vaccinated with Listeriaat showed no deleterious side effects. Even old mice (23 months old BALB/cByJ) and nude (nude/nude) mice that received numerous Listeriaat treatments, listeriosis or side effects were not observed (unpublished results). The highly attenuated Listeriaat used in this study is different from wild-type Listeria, in the sense that the latter multiplies in hepatocytes in the liver or epithelial cells of the gastrointestinal tract (Racz et al, 1972; Rosen and Gordon, 1987), whereas Listeriaat does not. It has also been shown in human clinical trials that Listeriaat-based cancer immunotherapy induce flu-like symptoms only (Maciag et al, 2009; Gravekamp and Paterson, 2010), whereas side effects of chemotherapy are known to be much more severe than flu-like symptoms.
In summary, we demonstrated that Listeriaat-based cancer immunotherapy was equally effective against metastatic breast cancer at young and old age, without having negative side effects. Our results strongly suggest that the intimate relationship between Listeriaat and MDSC contributed to the activation of T cells and NK cells at young and old age. Myeloid-derived suppressor cells also played an important role in selective delivery of Listeriaat to the TME, which may be useful to deliver anti-cancer agents selectively to the TME at young and old age. Although immune suppression in the TME is a major struggle in cancer immunotherapy at young and old age, the age-related T-cell unresponsiveness is an additional problem that is often ignored in human clinical trials. Therefore, tailoring cancer immunotherapy to older age may contribute to better clinical success.
Change history
11 June 2013
This paper was modified 12 months after initial publication to switch to Creative Commons licence terms, as noted at publication
References
Aslakson CJ, Miller FR (1992) Selective events in the metastatic process defined by analysis of the sequential dissemination of subpopulations of a mouse mammary tumor. Cancer Res 52 (6): 1399–1405.
Castro F, Leal B, Denny A, Bahar R, Lampkin S, Reddick R, Lu S, Gravekamp C (2009) Vaccination with Mage-b DNA induces CD8 T-cell responses at young but not old age in mice with metastatic breast cancer. Br J Cancer 101 (8): 1329–1337.
De Backer O, Verheyden AM, Martin B, Godelaine D, De Plaen E, Brasseur R, Avner P, Boon T (1995) Structure, chromosomal location, and expression pattern of three mouse genes homologous to the human MAGE genes. Genomics 28 (1): 74–83.
Diaz-Montero CM, Salem ML, Nishimura MI, Garrett-Mayer E, Cole DJ, Montero AJ (2009) Increased circulating myeloid-derived suppressor cells correlate with clinical cancer stage, metastatic tumor burden, and doxorubicin-cyclophosphamide chemotherapy. Cancer Immunol Immunother 58 (1): 49–59.
Extermann M (2011) Basic assessment of the older cancer patient. Curr Treat Options Oncol 12 (3): 276–285.
Gabrilovich DI, Nagaraj S (2009) Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol 9 (3): 162–174.
George AJ, Ritter MA (1996) Thymic involution with ageing: obsolescence or good housekeeping? Immunol Today 17 (6): 267–272.
Gravekamp C (2011) The impact of aging on cancer vaccination. Curr Opin Immunol 23 (4): 555–560.
Gravekamp C, Paterson Y (2010) Harnessing Listeria monocytogenes to target tumors. Cancer Biol Ther 9 (4): 1–9.
Grizzle WE, Xu X, Zhang S, Stockard CR, Liu C, Yu S, Wang J, Mountz JD, Zhang HG (2007) Age-related increase of tumor susceptibility is associated with myeloid-derived suppressor cell mediated suppression of T cell cytotoxicity in recombinant inbred BXD12 mice. Mech Ageing Dev 128 (11-12): 672–680.
Gunn GR, Zubair A, Peters C, Pan ZK, Wu TC, Paterson Y (2001) Two Listeria monocytogenes vaccine vectors that express different molecular forms of human papilloma virus-16 (HPV-16) E7 induce qualitatively different T cell immunity that correlates with their ability to induce regression of established tumors immortalized by HPV-16. J Immunol 167 (11): 6471–6479.
Hsieh CS, Macatonia SE, Tripp CS, Wolf SF, O’Garra A, Murphy KM (1993) Development of TH1 CD4+ T cells through IL-12 produced by Listeria-induced macrophages. Science 260 (5107): 547–549.
Ilkovitch D, Lopez DM (2009) The liver is a site for tumor-induced myeloid-derived suppressor cell accumulation and immunosuppression. Cancer Res 69 (13): 5514–5521.
Jemal A, Murray T, Ward E, Samuels A, Tiwari RC, Ghafoor A, Feuer EJ, Thun MJ (2005) Cancer statistics, 2005. CA Cancer J Clin 55 (1): 10–30.
Kim SH, Castro F, Gonzalez D, Maciag PC, Paterson Y, Gravekamp C (2008) Mage-b vaccine delivered by recombinant Listeria monocytogenes is highly effective against breast cancer metastases. Br J Cancer 99 (5): 741–749.
Kim SH, Castro F, Paterson Y, Gravekamp C (2009) High efficacy of a Listeria-based vaccine against metastatic breast cancer reveals a dual mode of action. Cancer Res 69 (14): 5860–5866.
Kim TS, Kang BY, Cho D, Kim SH (2003) Induction of interleukin-12 production in mouse macrophages by berberine, a benzodioxoloquinolizine alkaloid, deviates CD4+ T cells from a Th2 to a Th1 response. Immunology 109 (3): 407–414.
Lefebvre JS, Maue AC, Eaton SM, Lanthier PA, Tighe M, Haynes L (2012) The aged microenvironment contributes to the age-related functional defects of CD4 T cells in mice. Aging cell 11 (5): 732–740.
Li G, Smithey MJ, Rudd BD, Nikolich-Zugich J (2012) Age-associated alterations in CD8alpha+ dendritic cells impair CD8 T-cell expansion in response to an intracellular bacterium. Aging cell 11 (6): 968–977.
Lustgarten J, Dominguez AL, Thoman M (2004) Aged mice develop protective antitumor immune responses with appropriate costimulation. J Immunol 173 (7): 4510–4515.
Maciag PC, Radulovic S, Rothman J (2009) The first clinical use of a live-attenuated Listeria monocytogenes vaccine: a Phase I safety study of Lm-LLO-E7 in patients with advanced carcinoma of the cervix. Vaccine 27 (30): 3975–3983.
Miller RA (1996) The aging immune system: primer and prospectus. Science 273 (5271): 70–74.
Movahedi K, Guilliams M, Van den Bossche J, Van den Bergh R, Gysemans C, Beschin A, De Baetselier P, Van Ginderachter JA (2008) Identification of discrete tumor-induced myeloid-derived suppressor cell subpopulations with distinct T cell-suppressive activity. Blood 111 (8): 4233–4244.
Muraille E, Narni-Mancinelli E, Gounon P, Bassand D, Glaichenhaus N, Lenz LL, Lauvau G (2007) Cytosolic expression of SecA2 is a prerequisite for long-term protective immunity. Cell Microbiol 9 (6): 1445–1454.
Ostrand-Rosenberg S, Sinha P (2009) Myeloid-derived suppressor cells: linking inflammation and cancer. J Immunol 182 (8): 4499–4506.
Pan ZK, Weiskirch LM, Paterson Y (1999) Regression of established B16F10 melanoma with a recombinant Listeria monocytogenes vaccine. Cancer Res 59 (20): 5264–5269.
Portnoy DA, Auerbuch V, Glomski IJ (2002) The cell biology of Listeria monocytogenes infection: the intersection of bacterial pathogenesis and cell-mediated immunity. J Cell Biol 158 (3): 409–414.
Provinciali M, Argentati K, Tibaldi A (2000) Efficacy of cancer gene therapy in aging: adenocarcinoma cells engineered to release IL-2 are rejected but do not induce tumor specific immune memory in old mice. Gene Ther 7 (7): 624–632.
Provinciali M, Barucca A, Pierpaoli E, Orlando F, Pierpaoli S, Smorlesi A (2012) In vivo electroporation restores the low effectiveness of DNA vaccination against HER-2/neu in aging. Cancer Immunol Immunother 61 (3): 363–371.
Provinciali M, Smorlesi A, Donnini A, Bartozzi B, Amici A (2003) Low effectiveness of DNA vaccination against HER-2/neu in ageing. Vaccine 21 (9-10): 843–848.
Pylayeva-Gupta Y, Lee KE, Hajdu CH, Miller G, Bar-Sagi D (2012) Oncogenic Kras-induced GM-CSF production promotes the development of pancreatic neoplasia. Cancer Cell 21 (6): 836–847.
Racz P, Tenner K, Mero E (1972) Experimental Listeria enteritis. I. An electron microscopic study of the epithelial phase in experimental listeria infection. Lab Invest 26 (6): 694–700.
Rosen H, Gordon S (1987) Monoclonal antibody to the murine type 3 complement receptor inhibits adhesion of myelomonocytic cells in vitro and inflammatory cell recruitment in vivo. J Exp Med 166 (6): 1685–1701.
Seavey MM, Pan ZK, Maciag PC, Wallecha A, Rivera S, Paterson Y, Shahabi V (2009) A novel human Her-2/neu chimeric molecule expressed by Listeria monocytogenes can elicit potent HLA-A2 restricted CD8-positive T cell responses and impact the growth and spread of Her-2/neu-positive breast tumors. Clin Cancer Res 15 (3): 924–932.
Sharma S, Dominguez AL, Lustgarten J (2006) High accumulation of T regulatory cells prevents the activation of immune responses in aged animals. J Immunol 177 (12): 8348–8355.
Singh R, Dominiecki ME, Jaffee EM, Paterson Y (2005) Fusion to Listeriolysin O and delivery by Listeria monocytogenes enhances the immunogenicity of HER-2/neu and reveals subdominant epitopes in the FVB/N mouse. J Immunol 175 (6): 3663–3673.
Sinha P, Chornoguz O, Clements VK, Artemenko KA, Zubarev RA, Ostrand-Rosenberg S (2011) Myeloid-derived suppressor cells express the death receptor Fas and apoptose in response to T cell-expressed FasL. Blood 117 (20): 5381–5390.
Smith BD, Smith GL, Hurria A, Hortobagyi GN, Buchholz TA (2009) Future of cancer incidence in the United States: burdens upon an aging, changing. J Clin Oncol 27 (17): 2758–2765.
Utsuyama M, Hirokawa K, Kurashima C, Fukayama M, Inamatsu T, Suzuki K, Hashimoto W, Sato K (1992) Differential age-change in the numbers of CD4+CD45RA+ and CD4+CD29+ T cell subsets in human peripheral blood. Mech Ageing Dev 63 (1): 57–68.
Valenzuela J, Schmidt C, Mescher M (2002) The roles of IL-12 in providing a third signal for clonal expansion of naive CD8 T cells. J Immunol 169 (12): 6842–6849.
Weinberger B, Grubeck-Loebenstein B (2012) Vaccines for the elderly. Clin Microbiol Infect 18 (Suppl 5): 100–108.
Youn JI, Nagaraj S, Collazo M, Gabrilovich DI (2008) Subsets of myeloid-derived suppressor cells in tumor-bearing mice. J Immunol 181 (8): 5791–5802.
Zenewicz LA, Shen H (2007) Innate and adaptive immune responses to Listeria monocytogenes: a short overview. Microbes Infect 9 (10): 1208–1215.
Acknowledgements
This work was supported by NIA/NCI grant 1RO1 AG023096-01, training grant NIH1T32 Ag23475, and The Paul F Glenn Center for the Biology of Human Aging Research 34118A.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License.
Supplementary Information accompanies this paper on British Journal of Cancer website
Supplementary information
Rights and permissions
From twelve months after its original publication, this work is licensed under the Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/
About this article
Cite this article
Chandra, D., Jahangir, A., Quispe-Tintaya, W. et al. Myeloid-derived suppressor cells have a central role in attenuated Listeria monocytogenes-based immunotherapy against metastatic breast cancer in young and old mice. Br J Cancer 108, 2281–2290 (2013). https://doi.org/10.1038/bjc.2013.206
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bjc.2013.206
Keywords
This article is cited by
-
Camouflaging attenuated Salmonella by cryo-shocked macrophages for tumor-targeted therapy
Signal Transduction and Targeted Therapy (2024)
-
Bacteria-Mediated Synergistic Cancer Therapy: Small Microbiome Has a Big Hope
Nano-Micro Letters (2021)
-
Bacteria-cancer interactions: bacteria-based cancer therapy
Experimental & Molecular Medicine (2019)
-
Tumour-targeting bacteria engineered to fight cancer
Nature Reviews Cancer (2018)
-
White paper on microbial anti-cancer therapy and prevention
Journal for ImmunoTherapy of Cancer (2018)