Abstract
Endothelial dysfunction and impaired autophagic activity have a crucial role in aging-related diseases such as cardiovascular dysfunction and atherosclerosis. We have identified miR-216a as a microRNA that is induced during endothelial aging and, according to the computational analysis, among its targets includes two autophagy-related genes, Beclin1 (BECN1) and ATG5. Therefore, we have evaluated the role of miR-216a as a molecular component involved in the loss of autophagic function during endothelial aging. The inverse correlation between miR-216a and autophagic genes was conserved during human umbilical vein endothelial cells (HUVECs) aging and in vivo models of human atherosclerosis and heart failure. Luciferase experiments indicated BECN1, but not ATG5 as a direct target of miR-216a. HUVECs were transfected in order to modulate miR-216a expression and stimulated with 100 μg/ml oxidized low-density lipoprotein (ox-LDL) to induce a stress repairing autophagic process. We found that in young HUVECs, miR-216a overexpression repressed BECN1 and ATG5 expression and the ox-LDL induced autophagy, as evaluated by microtubule-associated protein 1 light chain 3 (LC3B) analysis and cytofluorimetric assay. Moreover, miR-216a stimulated ox-LDL accumulation and monocyte adhesion in HUVECs. Conversely, inhibition of miR-216a in old HUVECs rescued the ability to induce a protective autophagy in response to ox-LDL stimulus. In conclusion, mir-216a controls ox-LDL induced autophagy in HUVECs by regulating intracellular levels of BECN1 and may have a relevant role in the pathogenesis of cardiovascular disorders and atherosclerosis.
Similar content being viewed by others
Main
Aging represents a major risk factor for cardiovascular diseases including heart failure and atherosclerosis.1 Among the age-associated functional and structural changes, of particular note, is the decline in endothelial function and endothelial dysfunction has been demonstrated to be an early step in the development of atherosclerosis, contributing to the formation, progression and complications of atherosclerotic plaques.2 Therefore, the study of new target therapies to prevent or reverse this process represents a field of great interest. Autophagy an evolutionary conserved process involved in the degradation of long-lived proteins and excess of dysfunctional organelles, has recently been considered as a protective mechanism during the development of atherosclerosis because of its ability to stabilize plaques through the processing of oxidatively modified proteins, whereas acquired defects in plaques autophagy exacerbate atherosclerosis.3 Moreover, recent evidence suggests a role for autophagy in the maintenance of cardiac homeostasis and an imbalance in the autophagy regulation leads to the progression of heart failure.4 The age-associated reduction of autophagic activity has been reported5 and recent studies have revealed that the same signaling factors regulate both aging and autophagocytosis, thus highlighting the role of autophagy in the regulation of aging and age-related diseases.6 However, the underlying molecular mechanisms linking autophagy to endothelial dysfunction, atherosclerosis and myocardial infarction has not been fully explored. Oxidized low-density lipoprotein (ox-LDL) contributes greatly to the development and progression of atherosclerosis7 and previous studies found that ox-LDL can activate autophagy as a protective mechanism, leading to the degradation of ox-LDL through lysosomes in endothelial cells, vascular cells and macrophages.8, 9, 10 Cellular aging is a complex cell phenotype tightly controlled by specific gene expression programs11 and a prominent class of post-transcriptional regulators, defined microRNAs (miRNAs), has emerged in recent years. MiRNAs, a class of small, non-coding RNAs which interact with selected target mRNAs, repressing their expression, regulate endothelial dysfunction. To date, several miRNAs involved in endothelial cell function have been identified.12 MiRNAs have also been implicated in the full range of processes of cellular senescence, inflammation and cardiovascular diseases.13 We recently showed that miR-217 and miR-146a regulate senescence in human umbilical vein endothelial cells (HUVECs).14, 15 In the present study, we hypothesized that miR-216a, an miRNA induced during endothelial aging, also represents a new autophagy-related miRNA, acting through the regulation of its autophagy-promoting gene target, Beclin1 (BECN1). In particular, we aimed to evaluate whether miR-216a may inhibit ox-LDL-induced autophagy in HUVECs, leading to an impairment in ox-LDL degradation and implementing miR-216a as being crucial in atherogenesis and cardiovascular diseases.
Results
MiR-216a is upregulated and autophagy is impaired in aging HUVECs
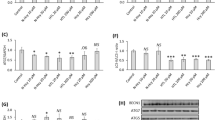
In a previous work, we have profiled the miRNA signature during HUVECs aging using a DNA Microarray approach, comparing samples from young cells (population doubling levels (PDLs) 8) versus old cells (PDLs44), according to an established endothelial senescence model. Obtained results indicated miR-216a as one of the miRNAs most induced with aging, and quantitative RT-PCR analyses confirmed the increase of miR-216a expression in old cells (n=10 per group) (Figure 1a).14 Computational miRNAs target analysis identified miR-216a potential target sequences in the 3′UTR regions of two essential autophagy genes: BECN1 and ATG5 (Supplementary Figure 1A), whose expression was accordingly downregulated in old HUVECs, as assessed by RT-PCR and western blot (Figures 1a and b). Moreover, the protein expression of the vesicle-associated form of the autophagic gene LC3B (microtubule associated protein 1 light chain 3) (LC3B-II), an important marker of autophagy, is reduced in older cells (Figure 1b), indicating that autophagic function decreases during endothelial senescence. To check whether sequences in BECN1- and ATG5-3′UTR are responsive to miR-216a, we used pGL3-BECN1-3′-UTR or pGL3-ATG5-3′UTR luciferase reporter vectors. Co-transfection of pre-miR-216a (miR216a), a specific RNA precursor which mimics mature miR-216a, with BECN1-3′-UTR reporter in HEK293 cells significantly decreased the relative luciferase activity compared with samples transfected with one reporter gene vector containing mutations in the predicted consensus sequences for miR-216a (1819–1825 bases in the sequence NM003766.3) (pGL3-BECN1mut-3′-UTR) (Supplementary Figure 1A). As expected, the nontargeting control scramble oligonucleotide did not have any effect on the reporter activity of both vectors (Figure 1c). These results demonstrated that the miR-216a binding sequences in the BECN1-3′-UTR is the region required for the miR-216a-mediated inhibition of BECN1 expression. On the contrary, in HEK293 cells transfected with ATG5-3′UTR reporter, there were no significant differences in the relative luciferase activities between cells co-transfected with miR216a or control oligonucleotide (Supplementary Figure 1B). In summary, these findings suggest that BECN1, but not ATG5, represents a direct target of miR-216a.
Autophagy is impaired in HUVECs during aging. (a) RT-PCR of endogenous miR-216a (n=10 per group), BECN1 and ATG5 mRNA levels (n=6) and (b) BECN1, ATG5 and LC3B protein levels (n=4) in HUVECs aging model. (**P<0.005, ***P<0.0005. Student’s t-test, data are mean ± S.E.M.). (c) miR-216a dose-dependent suppression of luciferase activity in HEK293 cells transfected with pGL3-BECN1-3′UTR vector containing miR-216a consensus binding sequences in the BECN1-3′-UTR, or pGL3-BECN1mut-3′UTR vector containing mutated miR-216a consensus binding sequences in the BECN1-3′-UTR (n=4). (**P<0.005, ***P<0.0005 miR-216a transfected cells compared with controls) by one-way ANOVA. Data are mean ± S.E.M.
The inverse correlation between miR-216a and autophagic genes is conserved in vivo in human cardiovascular diseases
Marker proteins of autophagy have been detected in atherosclerotic plaques and autophagy becomes dysfunctional with plaques progression.16 Therefore, we first analyzed 10 stable and 10 unstable atherosclerotic plaques from a previous study14 observing increased expression of miR-216a in unstable compared with stable plaques (n=10 per group) (Figure 2a); we next, investigated whether the miR-216a increase is associated with inhibition of autophagy during atherogenesis in vivo. To this end, we analyzed the expression of miR-216a and autophagic markers in a larger sample of human atherosclerotic plaques (n=65).
miR-216a and autophagy-related genes in atherosclerosis and heart failure. (a) Expression of miR-216a in unstable compared with stable plaques (n=10 per group) (*P=0.02. Student’s t-test, data are mean ± S.E.M.). (b) Correlation between miR-216a and BECN1 and ATG5 mRNA expression in human atherosclerotic plaques (n=65) (Spearman’s correlation test). (c) miR-216a expression in patients divided upon circulating LDL tertiles (LSD post-hoc analysis) D, BECN1 and ATG5 mRNA expression in myocardial biopsies from heart failure (HF) patients (n=13) compared with normal subjects (n=12) (*P<0.05, **P<0.01.Student’s t test, data are mean ± S.E.M.). (e) Correlation between miR-216a and BECN1 and ATG5 mRNA expression in myocardial biopsies (n=25) (Spearman’s correlation test)
Our results confirmed the negative correlation that we had found in HUVECs, between miR-216a and both BECN1 and ATG5 mRNA level (Figure 2b). To identify factors associated with miR-216a, we performed a linear regression analysis by including waist, sex, age and the use of statins as independent variables in the model. Among the variables included in the analysis, only LDL cholesterol levels (β=0.07, CI 95%=0.01–0.13, P=0.028) emerged as an independent and significant correlation factor (Table 1). Moreover, dividing patients into tertiles of plasma cholesterol LDL, we also found that miR-216a expression in atherosclerotic plaques is significantly increased in patients with higher LDL level compared with individuals with lower LDL levels (Figure 2c).
Greco et al.17 have recently shown that miR-216a, detectable in human endothelial cells but not in isolated mouse cardiac myocytes and fibroblasts, is significantly increased in myocardial biopsies from heart failure (HF) patients (n=13) compared with normal subjects (n=12). Here we show that in the same samples the mRNA expression of both BECN1 and ATG5 is reduced with HF (Figure 2d) and is negatively correlated with miR-216a level (Figure 2e), suggesting a role for endothelial miR-216a in regulating autophagy genes during HF.
The results of these experiments indicate that alterations in miR-216a expression can cause corresponding changes in autophagy in vivo in both atherosclerosis and myocardial infarction.
MiR-216a negatively regulates endogenous BECN1 expression in HUVECs
To obtain further experimental evidences supporting BECN1 as a target for miR-216a in endothelial cells, we examined the effect of miR-216a modulation on BECN1 expression. We found that overexpression of miR-216a in PDLs8 HUVECs significantly inhibited BECN1 expression both at mRNA and protein levels (Figures 3a and b). Interestingly, an indirect effect is also observed on ATG5 regulation; in fact ATG5 mRNA and protein expressions are reduced in miR-216a transfected cells compared with cells transfected with Co (Figures 3a and b). After transfection, miR-216a levels were determined by RT-PCR (Supplementary Figure 1C). Gene-expression studies revealed that miR-216a is also able to downregulate BECN1 in other models of endothelial cells such as HAEC and HCAEC (Figure 3c). Knockdown of endogenous miR-216a with a specific antisense miR-oligonucleotide (A216a) in PDLs44 significantly increased BECN1 and ATG5 mRNA and protein expression (Figures 3d and e). Autophagy activation, evaluated by LC3B analysis is not affected by miR-216a modulation in both young and old cells (data not shown).
miR-216a regulates the expression of BECN1 in endothelial cells. (a) BECN1 and ATG5 mRNA and (b) protein expression in PDLs8 HUVECs transfected with miR216a or scramble M control (n=4). (c) BECN1 mRNA expression in PDLs8 HAECs and HCAEC transfected with miR-216a or scramble M oligo (n=4). (d) BECN1 and ATG5 mRNA and e, protein expression in PDLs44 HUVECs transfected with A216a or scramble A control (n=4). (*P<0.05, **P<0.01. Student’s t-test, data are mean ± S.E.M.)
MiR-216a overexpression inhibits ox-LDL-induced autophagy in HUVECs
BECN1 has an essential role in activating autophagy. To determine the functional consequence of its modulation by miR-216a in HUVECs, we tested the effect of miR-216a on autophagic response to stress-induced endothelial dysfunction in young endothelial cells. Ox-LDL is a major risk factor in the development of atherosclerosis and plasma level of ox-LDL shows a good correlation with HF severity and mortality. Oxidized lipids have been demonstrated to trigger autophagy in endothelial cells as a protective mechanism to prevent early processes of atherosclerosis.7, 8, 18 Therefore we investigated whether miR-216a expression may interfere with the autophagy activity induced by ox-LDL treatment in PDLs8 HUVECs. First, we found that in PDLs8 HUVEC ox-LDL (100 μg/ml, 16 h) stimulated cells, miR-216a expression is significantly downregulated compared with control cells (Figure 4a). Moreover our results confirmed that treatment of young cells with ox-LDL is able to activate autophagy, as evidenced by the increases in BECN1, ATG5, LC3B mRNA and protein amounts (Figures 4b and c). Notably, PDLs8 HUVECs transfected with miR-216a showed a blunted autophagic response to ox-LDL, as evaluated by a significant reduction in BECN1, ATG5, LC3B mRNA and protein expressions (Figures 4b and c). To establish the relevance of the present findings to other endothelial models, we analyzed the expression of miR-216a during aging in human aortic endothelial cells (HAECs) finding an increase of miR-216a in PDLs44 HAECs compared with PDLs8 cells (Supplementary Figure 2A). In young HAECs, miR-216a expression is also downregulated in ox-LDL treated cells (Supplementary Figure 2B) and the overexpression of miR-216a leads to a reduction of BECN1, ATG5 and LC3B mRNA in the presence of ox-LDL (Supplementary Figure 2C), suggesting that the mechanism of miR-216a regulation of autophagy is conserved in different models of endothelial cells.
miR-216a regulation of ox-LDL-induced autophagy genes in PDLs8 HUVECs. (a) Endogenous miR-216a expression in PDLs8 HUVECs treated with ox-LDL (100 μg/ml, 16 h) (*P<0.05 Student’s t-test, data are mean ± S.E.M.). PDLs8 HUVECs transfected with miR216a and treated with ox-LDL were analyzed for: (b and c), BECN1, ATG5 and LC3B mRNA (n=6) and protein expression (n=4). (*P<0.05, **P<0.005, ***P<0.0005. one-way ANOVA. Data are mean ± S.E.M.)
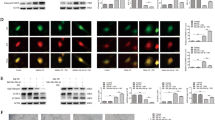
To confirm the inhibition of ox-LDL-induced autophagy by miR-216a, we analyzed the autophagic activity in live cells by a flow cytometric assay and found that in ox-LDL-exposed PDLs8 HUVECs, miR216a overexpression reduced the percentage of autophagy by almost 40% compared with the ox-LDL cells transfected with the control scramble oligo (Figure 5a). To further confirm this result, we analyzed LC3B processing and LC3B-II accumulation in young HUVECs. Fluorescence microscopy revealed a punctuate pattern of endogenous LC3B, indicating the processing of soluble LC3B-I to membrane-bound LC3B-II in autophagic vacuoles in ox-LDL-exposed cells, whereas the overexpression of miR-216a inhibited the formation of LC3B-II positive dots in presence of ox-LDL (Figure 5b). Conversely to its effect on ox-LDL-induced autophagy, overexpression of miR-216a in young cells did not affect the autophagic process induced by starvation, as evidenced by LC3B mRNA levels, and endogenous punctuate pattern of LC3 (Supplementary Figures 3A and B), indicating a specific role for miR-216a in atherogenic stress response.
miR-216a overexpression blocked ox-LDL-induced autophagy and regulates ox-LDL accumulation and monocytes adhesion in PDLs8 HUVECs. (a) Cyto-ID autophagy detection expressed as a percentage of total viable cells (n=6). (b) LC3B immunofluorescence showing LC3B-II positive signals with punctuate structures in control cells under ox-LDL treatment and a reduction in ox-LDL miR216a transfected cells. Scale bar, 60X. Fluorescence intensity from experiment derived from average of 35 cells in four images per group. (**P<0.005, one-way ANOVA. Data are mean ± S.E.M.). PDLs8 HUVECs transfected with miR-216a and treated with Dil-ox-LDL or ox-LDL were analyzed for: (c) Dil-ox-LDL fluorescence (***P=0.0002 Student’s t-test, data are mean ± S.E.M.) and d, THP-1 monocyte adhesion. (*P<0.05, **P<0.005, one-way ANOVA. Data are mean ± S.E.M.)
MiR-216a induces ox-LDL accumulation and monocyte adhesion in HUVECs
Previous reports indicated that inhibition of autophagy induces ox-LDL accumulation in HUVECs.8 Therefore, to evaluate whether the inhibitory effect of miR-216a on ox-LDL-induced autophagic response has a role in the control of endothelial function, we performed experiments using dioctadecyl-tetramethylindo-carbocyanine perchlorate (Dil)–labeled oxidized LDL (Dil-oxLDL) (100 ng/ml, 16 h), ox-LDL labeled with a fluorescent probe, and showed that miR-216a overexpression induced ox-LDL accumulation in PDLs8 HUVECs, suggesting that miR-216a inhibition of ox-LDL-induced autophagy is involved in the uptake and degradation of ox-LDL (Figure 5c). As modulation of monocyte adhesion onto ox-LDL-activated endothelium could represent a miR-216a atherogenic mechanism in the early stage of atherosclerosis, we performed an in vitro adhesion assay using THP-1 monocytes stained with 5- and 6-carboxyfluorescein diacetate (CFDA-SE). Obtained data indicated a marked increase in THP-1 adherence to the ox-LDL-activated young HUVECs overexpressing miR-216a (Figure 5d).
Downregulation of miR-216a protects from ox-LDL accumulation and monocyte adhesion in old HUVECs
Unlike young cells, old HUVECs showed a significant increase of miR-216a in response to ox-LDL stimulation (Figure 6a). To test whether silencing endogenous miR-216a in old HUVECs would have a positive impact on endothelial function, we transfected A216a in PDLs44 HUVECs and checked the effect in the presence of ox-LDL. Our results showed that miR-216a inhibition in ox-LDL exposed old cells resulted in a significant increase of LC3B punctuate (Figure 6b) and reduction of ox-LDL uptake (Figure 6c) and THP-1 adhesion (Figure 6d), indicating an increase in autophagic activity that may exert a protective anti atherogenic role against ox-LDL treatment.
miR-216a inhibition reduces ox-LDL accumulation and monocytes adhesion on PDLs44 HUVECs. (a) Endogenous miR-216a expression in PDLs44 HUVECs treated with ox-LDL (100 μg/ml, 16 h) (*P<0.03 Student’s t-test, data are mean ± S.E.M.). PDLs44 HUVECs transfected with A216a and treated with ox-LDL or Dil-ox-LDL were analyzed for: (b) LC3B immunofluorescence ( ***P<0.0005, one-way ANOVA. Data are mean ± S.E.M.), (c) Dil-ox-LDL fluorescence (***P<0.0001 Student’s t test, data are mean ± S.E.M.), d, THP-1 monocyte adhesion. (*P<0.05, one-way ANOVA. Data are mean ± S.E.M.). (n=4 for each experiment)
Discussion
Diminished autophagic activity has a major role in several aging-related disorders.19 In this study, we first demonstrated that key autophagy-related genes, such as BECN1, ATG5 and LC3B-II, are decreased during HUVEC aging, suggesting, as already reported in other cell lines, that the autophagic process is impaired with aging at multiple steps of the pathway.20 Moreover, we identified miR-216a as an miRNA induced during endothelial aging that is able to directly reduce the expression of BECN1, leading to an indirect downregulation of ATG5. BECN1 controls the early stages of autophagic vesicle formation and it was previously shown that inhibition of BECN1 blocked autophagy in many experimental systems.21 Therefore BECN1 cellular levels are critical for the activation of the canonical autophagy pathways. Other miRNAs, such as miR-376b and miR-30a, were recently shown to have a role in autophagy regulation in different cellular models, in particular through the regulation of BECN1.21, 22, 23 However, in our cellular model, we could not find any modulation of those specific miRNAs during endothelial aging.
The in vitro data could be also translated in vivo; in fact, we showed that miR-216a is expressed in atherosclerotic plaques obtained from patients who underwent carotid endarterectomy for symptomatic disease and is negatively correlated with both, BECN1 and ATG5 mRNA expression. Moreover, LDL cholesterol levels are independent predictors of high miR-216a expression even after adjusting for potential confounders such as aging, gender, abdominal adiposity and the use of drugs affecting LDL cholesterol levels (statins). Dyslipidemia is known to be closely associated with atherosclerosis.24 Several studies have shown that LDL cholesterol and total cholesterol concentrations are related to ox-LDL measured by different antibodies.25 Oxidation of LDL and its transfer across the endothelium into the arterial wall are crucial steps in the atherogenic process and are probably affected by the susceptibility of LDL to oxidation, the particle size, and the amount of LDL in the circulation, the composition of LDL and local oxidative stress in the arterial wall.23 Ox-LDL levels are significantly increased in subjects with various dyslipidemias and coronary artery disease and the concentrations of serum LDL cholesterol represents an independent determinant of ox-LDL, thus being one of the most important factors contributing to the generation of circulating ox-LDL.26 Therefore, we can speculate that LDL cholesterol levels reflect an increase of ox-LDLs that in turn may be related to miR-216a expression and regulation.
Limitations to our findings in atherosclerotic plaques are due to the evidence that detection of autophagy in plaques is problematic and good biological markers are still needed to better determine the involved cell types and the associations with early or late lesions.
The role of miR-216a in cardiovascular system is also proven by the evidence that it is significantly increased in myocardial biopsies from patients with ischemic heart failure, compared with normal subjects. In addition, miR-216a expression was already demonstrated to be detectable in human endothelial cells but not in isolated mouse cardiac myocytes and fibroblasts suggesting that it might take part in the physiological and pathophysiological vascular processes.17 Confirming in another model in vivo the data obtained in atherosclerotic plaques, we found that autophagy is downregulated in heart failure and is inversely correlated to miR-216a expression. Coronary atherosclerosis is the main cause of heart failure and myocardial endothelial cells have a crucial role in regulating and maintaining cardiac function. Endothelial dysfunction in the heart has been described after MI, ischemia-reperfusion injury and in failing hearts.27, 28, 29 Oxidative stress levels may have a significant role in the pathogenesis of heart failure, and plasma levels of ox-LDL show a good correlation with heart failure severity and mortality.18 Moreover, a significant negative correlation exists between the plasma level of ox-LDL and left ventricular ejection fraction (LVEF).30 Interestingly, miR-216a expression was also inversely correlated with LVEF, indirectly suggesting an miR-216a activation in presence of high levels of ox-LDL. The extent of adverse myocardial remodeling contributes essentially to the prognosis after myocardial infarction and strategies aimed at inducing autophagy represent novel potential therapeutic approach to limit infarction size and to attenuate adverse left ventricular remodeling following myocardial infarction. Autophagy activation, especially in the border zone of the infarcted myocardium, limits infarct size.31 MiR-216a has been found to be further increased in the border zone compared with remote in the myocardial biopsies from heart failure patients, suggesting a link with autophagy impairment in severe disease. Due to the in vivo relevance of miR-216a in cardiovascular diseases, we investigated its role in a cellular model of endothelial dysfunction. In young endothelial cells, miR-216a expression led to an impairment of autophagy activation induced by an atherogenic stimulus, such as ox-LDL, leading to an increase in ox-LDL accumulation and monocyte adhesion; features resembling an aging phenotype. Interestingly, in old endothelial cells, the restoration of this component of the autophagy pathway through miR-216a inhibition could improve the autophagic functions in response to ox-LDL, resulting in a delay of the cellular aging process. MiR-216a is significantly reduced by ox-LDL in young endothelial cells, whereas it is increased by ox-LDL in old endothelial cells, suggesting the activation of different regulatory systems with aging in response to ox-LDL and that dynamically changing environmental modifications may have great potential to influence ox-LDL-mediated signaling and subsequent response during atherogenesis and cardiovascular diseases. In addition, we found that the autophagy induced by starvation was not affected by miR-216a modulation, indicating a specific role for miR-216a in response to vascular damage. On the contrary, miR376b, targeting BECN1, is involved in the regulation of starvation-induced autophagy in cancer cells.21 Because several miRNA sequences are recognizable in BECN1-3′UTR region and considering the crucial role of BECN1 in the autophagy activation, it is conceivable that different conditions and cellular events affect the expression of specific miRNAs regulating BECN1 and thereby control autophagy.
Aberrant expression of miRNAs is known to act on many pathophysiological processes, including endothelial dysfunction. There have been many studies focusing on miRNAs function in the vasculature and the heart.32, 33 However, few reports have concentrated on the effect of miRNAs on autophagy gene expression in a context different from neoplastic disease. Our study indicates that miR-216a might have very important roles in aging-related cardiovascular diseases including atherosclerosis and heart failure. To our knowledge, this is the first report demonstrating that one microRNA may represent a connection between autophagy and endothelial dysfunction. Considering that both are important responses to oxidative stress and that their dysregulation has been implicated in aging, atherosclerosis and heart failure, our finding could help to gain a better understanding of the pathogenesis, prevention and treatment of cardiovascular aging-related diseases.
Materials and Methods
Cells and cell culture
Human umbilical vein endothelial cells (HUVECs), human aortic endothelial cells (HAECs), and human coronary artery endothelial cells (HCAECs) were purchased from Lonza (Basel, Switzerland) and cultured as described previously.34 Population-doubling levels (PDLs) were calculated as described previously;35 briefly, the number of population doublings (PD) that occurred between passages was calculated according to the equation PD=log2(Ch/Cs), where Ch is the number of viable cells at harvest and Cs is the number of cells seeded. All experiments were performed at the PDLs indicated in the text. Cells were incubated at 37 °C, 16 h, in the presence of 100 μg/ml ox-LDL (Biomedical Technologies Inc., Stoughton, MA, USA).
miRNA and mRNA real-time quantitative reverse-transcription polymerase chain reaction analysis
Real-time quantification to measure miRNAs was performed in samples obtained by using mirVana miRNA isolation kit according to the instructions (Ambion Inc, Austin, TX, USA) with the TaqMan miRNA reverse transcription kit and miRNA assay according to the manufacturer’s protocol with the ABI PRISM 7000 system (Applied Biosystems, Foster City, CA, USA). The U43 small nucleolar RNA (RNU43) was used as the housekeeping small RNA reference gene. For mRNA analysis, single-strand complementary DNA (cDNA) was synthesized from 1 μg of total RNA sample isolated through TRIzol reagent (Invitrogen, Carlsbad, CA, USA) with a high-capacity cDNA archive kit according to the standard protocol. Fifty nanograms of cDNA was amplified by real-time polymerase chain reaction (RT-PCR) and normalized to 18S ribosomal RNA as an endogenous control. Each reaction was performed in triplicate, and analysis was performed by the 2−ΔΔCt method as described previously.36
Western blot analysis
Total lysates were subjected to SDS-PAGE as described previously.14 The following antibodies were used: BECN1, ATG5, LC3B (Abcam Inc, Cambridge, MA, USA), and tubulin (Sigma, St Louis, MO, USA).
Luciferase assay
The pGL3-BECN1-3′-UTR, pGL3-ATG5-3′-UTR and pGL3-BECN1mut-3′-UTR vectors were obtained from Origene Technologies Inc. (Rockville, MD, USA). Each vector, along with varying doses (20 and 40 nmol/l) of miRIDIAN miR-216a mimic (MiR216a) or 40 nmol/l of control scramble sequence specific for mimic (scramble M) (Dharmacon, Lafayette, CO, USA), was transfected into human embryonic kidney 293 (HEK293) cells with Lipofectamine 2000 reagent (Invitrogen) according to the manufacturer’s instructions. Cells were cultured for 2 days and assayed with the Dual-Luciferase Reporter Assay System (Promega, Madison, WI, USA).
Atherosclerotic plaque sampling and histology
Frozen human atherosclerotic plaque samples (n=65) from patients who underwent carotid endarterectomy for symptomatic disease were homogenized with a polytron homogenizer in TRIzol reagents (Invitrogen) for total RNA isolation. Real-time PCR analyses were performed as described above. Subject characteristics and treatments are described in Supplementary Table 1. Atherosclerotic plaque histology was performed as previously described.37 The study was approved by the ethics committee, and subjects provided informed written consent for the use of atherosclerotic material for research use. All procedures were performed according to the Declaration of Helsinki.
Myocardial biopsies
Left ventricular cardiac biopsies were derived from consecutive patients affected by dilated hypokinetic ischemic cardiomyopathy and obtained during surgical ventricular restoration procedures performed as described previously.38 Biopsies were harvested from the nonischemic, remote myocardium and processed as described previously.17 Briefly, samples were immediately immersed in RNAlater (QIAGEN GmbH) and stored at 4°C for no more than 24 h before RNA extraction. Heart failure patients (HF) (n=13, see Supplementary Table 2 for clinical features) were sex- and age-matched with controls (CTR), subjects who died from causes other than stroke, ischemia or cachexia for chronic diseases (n=12, three females/nine males, aged 61.4±14.5 years). Bioptic specimens were taken after informed consent disclosing future use for research. The investigation conformed to the principles outlined in the Helsinki Declaration and to Italian laws and guidelines and was authorized by a local ethics committee.
miRNA transfection
Nucleofection of HUVECs was performed according to the manufacturer’s instructions with the Nucleofector machine (Amaxa/Lonza, Basel, Switzerland). Cells (1 × 106) were resuspended in 100 μl of HUVEC nucleofection solution (Amaxa/Lonza), and 20 nmol/l of miRIDIAN miR-216a mimic (miR216a), 20 nmol/l of miRIDIAN mir-216a inhibitor (A216a), or 20 nmol/l of control scramble sequences specific for mimic (scramble M) or inhibitor (scramble A) experiments (Dharmacon, Lafayette, CO, USA) was added. Samples were transferred into certified cuvettes (Amaxa/Lonza) and transfected with program A-034. Fresh medium (500 μl) was added immediately after transfection to each cuvette, and the cells were plated and incubated at 37 °C for 3 days.
Adhesion of monocytes to oxidized LDL-activated endothelial cells
THP-1 monocytes were washed in PBS and were then labeled for 15 min with CFDA-SE (Molecular Probes for Life Technologies, Carlsbad, CA, USA). At the end, cells were re-suspended in fresh medium and were incubated for 30 min at 37 °C. Labeled THP-1 were added (for 1 h, at 37 °C) to unactivated or oxidized LDL-activated (ox-LDL 100 μg/ml, 16 h, Biomedical Technologies Inc.) HUVEC monolayers. THP-1 adhesion was visualized using an inverted microscope.
Immunofluorescence
HUVEC cells, after various treatments, were washed in PBS and fixed for 15 min with 4% paraformaldehyde, permeabilized with Methanol for 10 min at −20 °C and blocked in PBS containing 5% normal goat serum and 0.3% Triton-X-100 for 1 h at RT. Primary antibody against LC3B (Cell Signaling) was used overnight at 4 °C in PBS containing 1% of BSA and 0.3% Triton. Anti-goat IgG antibody conjugated to Alexa Fluor 488 was used for 1 h at RT then rinsed several times; cells cultures on glass slides were mounted with Vectashield mounting medium containing DAPI (Molecular Probes), and analyzed with a confocal microscope (Nikon Inc., Melville, NY, USA). Images were digitally analyzed to quantify the fluorescence intensity of cells.
Dil-ox-LDL incorporation study
Adherent cells were stained for the uptake of Dil-ox-LDL (Biomedical Technologies Inc.). Cells were incubated at 37 °C in the presence of 100 ng/ml Dil-ox-LDL overnight. HUVECs were then washed three times and fixed for 15 min in PBS containing 4% paraformaldehyde. The number of total attached cells was evaluated by DAPI staining. Cells and Dil-ox-LDL incorporation were visualized using a laser confocal microscope (Nikon Inc.). Fifteen or more random microscopic fields were examined to quantify the fluorescence intensity of Dil-ox-LDL incorporation.
Cyto-ID autophagy detection assay
HUVEC cells were maintained in culture and treated with compound of interest. At the end of each treatment 0.5 × 106 cells were trypsinized, washed and re-suspended in freshly diluted Cyto-ID green Detection reagent as directed by the manufacturer (Cyto-ID Autophagy Detection kit, Enzo Life Sciences, Farmingdale, NY, USA). Samples were incubated at RT for 30 min and analyzed without washing in the green channel (FL1) of a FACSCalibur flow cytometer (BD Biosciences, San Jose, CA, USA). Results were expressed as percentage of Cyto-ID positive cells of total live cells.
Statistical analysis
Quantitative variables were expressed as means (± S.D.). Categorical variables were presented as percentages. Each quantitative variable was checked for normality of distribution by the Kolmogorov–Smirnov test. The Mann–Whitney test was used for variables with non-normal distributions. The significance of difference between percentages in groups was evaluated with the χ2-test. ANOVA univariate with LSD post-hoc analysis was used to compare inflammatory, biochemical, and clinical parameters among patients divided in LDL cholesterol tertiles.
Correlation coefficients were used to describe simple relationships between variables. We then used a multiple linear regression analysis to explore independent association between miR-216a – entered into the model as the dependent variable – and age, sex, waist and statins used as independent variables.
Student t-test or one-way analysis of variance (ANOVA) were used as appropriate. A P value <0.05 was considered statistically significant. All analyses were performed with GraphPad Prism 5.0 (GraphPad, San Diego, CA, USA). a.u. indicates arbitrary units.
Abbreviations
- BECN1:
-
Beclin1
- HUVECs:
-
human umbilical vein endothelial cells
- ox-LDL:
-
oxidized low-density lipoprotein
- LC3B:
-
microtubule associated protein 1 light chain 3
- miRNA:
-
microRNA
- PDLs:
-
population doubling levels
- HF:
-
heart failure
- miR216a:
-
pre-miR-216a
- A216a:
-
mir-216a inhibitor
- CFDA-SE:
-
5-(and-6)-carboxyfluorescein diacetate
- Dil-oxLDL:
-
dioctadecyl-tetramethylindo-carbocyanine perchlorate (Dil)–labeled oxidized LDL
- HAECs:
-
human aortic endothelial cells
- HCAECs:
-
human coronary artery endothelial cells
References
North BJ, Sinclair DA . The intersection between aging and cardiovascular disease. Circ Res 2012; 110: 1097–1108.
Davignon J, Ganz P . Role of endothelial dysfunction in atherosclerosis. Circulation 2004; 109: III27–III32.
Martinet W, De Meyer GR . Autophagy in atherosclerosis: a cell survival and death phenomenon with therapeutic potential. Circ Res 2009; 104: 304–317.
Gustafsson ÅB, Gottlieb RA . Autophagy in ischemic heart disease. Circ Res. 2009; 104: 150–158.
Cuervo AM . Autophagy and aging: keeping that old broom working. Trends Genet 2008; 24: 604–612.
Salminen A, Kaarniranta K . Regulation of the aging process by autophagy. Trends Mol Med 2009; 15: 217–224.
Lewis A . Oxidative modification of LDL and atherogenesis.Steinberg D. Circulation 1997; 95: 1062–1071.
Zhang YL, Cao YJ, Zhang X, Liu HH, Tong T, Xiao GD et al. The autophagy-lysosome pathway: a novel mechanism involved in the processing of oxidized LDL in human vascular endothelial cells. Biochem Biophys Res Commun 2010; 394: 377–382.
Muller C, Salvayre R, Nègre-Salvayre A, Vindis C . HDLs inhibit endoplasmic reticulum stress and autophagic response induced by oxidized LDLs. Cell Death Differ 2011; 18: 817–828.
Ding Z, Wang X, Schnackenberg L, Khaidakov M, Liu S, Singla S et al. Regulation of autophagy and apoptosis in response to ox-LDL in vascular smooth muscle cells, and the modulatory effects of the microRNA hsa-let-7g. Int J Cardiol 2013; 168: 1378–1385.
Fridman AL, Tainsky MA . Critical pathways in cellular senescence and immortalization revealed by gene expression profiling. Oncogene 2008; 27: 5975–5987.
Urbich C, Kuehbacher A, Dimmeler S . Role of microRNAs in vascular diseases, inflammation, and angiogenesis. Cardiovasc Res 2008; 79: 581–588.
Schroen B, Heymans S . Small but smart—microRNAs in the centre of inflammatory processes during cardiovascular diseases, the metabolic syndrome, and ageing. Cardiovasc Res 2012; 93: 605–613.
Menghini R, Casagrande V, Cardellini M, Martelli E, Terrinoni A, Amati F et al. MicroRNA 217 modulates endothelial cell senescence via silent information regulator 1. Circulation 2009; 120: 1524–1532.
Vasa-Nicotera M, Chen H, Tucci P, Yang AL, Saintigny G, Menghini R et al. miR-146a is modulated in human endothelial cell with aging. Atherosclerosis 2011; 217: 326–330.
Razani B, Feng C, Coleman T, Emanuel R, Wen H, Hwang S et al. Autophagy links inflammasomes to atherosclerotic progression. Cell Metab 2012; 15: 534–544.
Greco S, Fasanaro P, Castelvecchio S, D'Alessandra Y, Arcelli D, Di Donato M et al. MicroRNA Dysregulation in Diabetic Ischemic Heart Failure Patients. Diabetes 2012; 61: 1633–1641.
Tsutsui T, Tsutamoto T, Wada A, Maeda K, Mabuchi N, Hayashi M et al. Plasma oxidized low-density lipoprotein as a prognostic predictor in patients with chronic congestive heart failure. J Am Coll Cardiol 2002; 39: 957–962.
Rubinsztein DC, Mariño G, Kroemer G . Autophagy and aging. Cell 2011; 146: 682–695.
Kang HT, Lee KB, Kim SY, Choi HR, Park SC . Autophagy impairment induces premature senescence in primary human fibroblasts. PLoS One 2011; 6: e23367.
Korkmaz G, le Sage C, Tekirdag KA, Agami R, Gozuacik D . miR-376b controls starvation and mTOR inhibition-related autophagy by targeting ATG4C and BECN1. Autophagy 2012; 8: 165–176.
Zhu H, Wu H, Liu X, Li B, Chen Y, Ren X et al. Regulation of autophagy by a beclin 1-targeted microRNA, miR-30a, in cancer cells. Autophagy 2009; 5: 816–823.
Pan W, Zhong Y, Cheng C, Liu B, Wang L, Li A et al. MiR-30-regulated autophagy mediates angiotensin II-induced myocardial hypertrophy. PLoS One 2013; 8: e53950.
Brown MS, Goldstein JL . Lipoprotein metabolism in the macrophage: implications for cholesterol deposition in atherosclerosis. Annu Rev Biochem 1983; 52: 223–261.
Liu ML, Ylitalo K, Salonen R, Salonen JT, Taskinen MR . Circulating oxidized low-density lipoprotein and its association with carotid intima-media thickness in asymptomatic members of familial combined hyperlipidemia families. Arterioscler Thromb Vasc Biol 2004; 24: 1492–1497.
Wu J, Shi YH, Niu DM, Li HQ, Zhang CN, Wang J . Association among retinol-binding protein 4, small dense LDL cholesterol and oxidized LDL levels in dyslipidemia subjects. J Clin Biochem 2012; 45: 619–622.
Boak L, Chin-Dusting JP . Hypercholesterolemia and endothelium dysfunction: role of dietary supplementation as vascular protective agents. Curr Vasc Pharmacol 2004; 2: 45–52.
MacCarthy PA, Shah AM . Impaired endothelium-dependent regulation of ventricular relaxation in pressure-overload cardiac hypertrophy. Circulation 2000; 101: 1854–1860.
Fraccarollo D, Widder JD, Galuppo P, Thum T, Tsikas D, Hoffmann M et al. Improvementin left ventricular remodeling by the endothelial nitric oxide synthase enhancerAVE9488 after experimental myocardial infarction. Circulation 2008; 118: 818–827.
Singal PK, Kapur N, Dhillon KS, Beamish RE, Dhalla NS . Role of free radicals in catecholamine-induced cardiomyopathy. Can J Physiol Pharmacol 1982; 60: 1390–1397.
Buss SJ, Muenz S, Riffel JH, Malekar P, Hagenmueller M, Weiss CS et al. Beneficial effects of Mammalian target of rapamycin inhibition on left ventricular remodeling after myocardial infarction. J Am Coll Cardiol 2009; 54: 2435–2446.
Menghini R, Casagrande V, Federici M . MicroRNAs in Endothelial Senescence and Atherosclerosis. J Cardiovasc Transl Res 2013; 6: 924–930.
Tijsen AJ, Pinto YM, Creemers EE . Non-cardiomyocyte microRNAs in heart failure. Cardiovasc Res 2012; 93: 573–582.
Federici M, Pandolfi A, De Filippis EA, Pellegrini G, Menghini R, Lauro D et al. G972R IRS-1 variant impairs insulin regulation of endothelial nitric oxide synthase in cultured human endothelial cells. Circulation 2004; 109: 399–405.
Maciag T, Hoover GA, Stemerman MB, Weinstein R . Serial propagation of human endothelial cells in vitro. J Cell Biol 1981; 91: 420–426.
Menghini R, Menini S, Amoruso R, Fiorentino L, Casagrande V, Marzano V et al. Tissue inhibitor of metalloproteinase 3 deficiency causes hepatic steatosis and adipose tissue inflammation in mice. Gastroenterology 2009; 136: 663–672.
Mauriello A, Sangiorgi GM, Virmani R, Trimarchi S, Holmes DR Jr, Kolodgie FD et al. A pathobiologic link between risk factors profile and morphological markers of carotid instability. Atherosclerosis 2010; 208: 572–580.
Castelvecchio S, Menicanti L, Donato MD . Surgical ventricular restoration to reverse left ventricular remodeling. Curr Cardiol Rev 2010; 6: 15–23.
Acknowledgements
No other persons besides the authors have made substantial contributions to this manuscript. This manuscript was in part funded by Fondazione Roma Grant 2008, PRIN 2009FATXW3_002, FP-7 EURHYTHDIA and Associazione Italiana per la Ricerca sul Cancro (Grant AIRC IG-13163) to MF, FM and SG are supported by Ministero della Salute and Associazione Italiana per la Ricerca sul Cancro (Grant AIRC IG-11436).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Edited by G Melino
Supplementary Information accompanies this paper on Cell Death and Disease website
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/3.0/
About this article
Cite this article
Menghini, R., Casagrande, V., Marino, A. et al. MiR-216a: a link between endothelial dysfunction and autophagy. Cell Death Dis 5, e1029 (2014). https://doi.org/10.1038/cddis.2013.556
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/cddis.2013.556
Keywords
This article is cited by
-
The SGLT2 inhibitor empagliflozin attenuates atherosclerosis progression by inducing autophagy
Journal of Physiology and Biochemistry (2024)
-
High-density lipoprotein regulates angiogenesis by affecting autophagy via miRNA-181a-5p
Science China Life Sciences (2024)
-
Spermidine improves angiogenic capacity of senescent endothelial cells, and enhances ischemia-induced neovascularization in aged mice
Scientific Reports (2023)
-
The lncRNA TCONS_00021785/miR-21-5p/Trim33 axis regulates VMP1-mediated zymophagy, reduces the activation of trypsinogen, and promotes acinar cell recovery
Cell Death Discovery (2022)
-
Autophagy and apoptosis cascade: which is more prominent in neuronal death?
Cellular and Molecular Life Sciences (2021)