Abstract
In muscle tissue the protein caveolin-3 forms caveolae – flask-shaped invaginations localized on the cytoplasmic surface of the sarcolemmal membrane. Caveolae have a key role in the maintenance of plasma membrane integrity and in the processes of vesicular trafficking and signal transduction. Mutations in the caveolin-3 gene lead to skeletal muscle pathology through multiple pathogenetic mechanisms. Indeed, caveolin-3 deficiency is associated to sarcolemmal membrane alterations, disorganization of skeletal muscle T-tubule network and disruption of distinct cell-signaling pathways. To date, there have been 30 caveolin-3 mutations identified in the human population. Caveolin-3 defects lead to four distinct skeletal muscle disease phenotypes: limb girdle muscular dystrophy, rippling muscle disease, distal myopathy, and hyperCKemia. In addition, one caveolin-3 mutant has been described in a case of hypertrophic cardiomyopathy. Many patients show an overlap of these symptoms and the same mutation can be linked to different clinical phenotypes. This variability can be related to additional genetic or environmental factors. This review will address caveolin-3 biological functions in muscle cells and will describe the muscle and heart disease phenotypes associated with caveolin-3 mutations.
Similar content being viewed by others
In brief
-
In skeletal muscle tissue, the membrane protein Caveolin-3 regulates sarcolemmal stability and modulates the activity of different signalling pathways.
-
In vitro and in vivo experimental models indicate that maintenance of physiological levels of Caveolin-3 is essential for normal skeletal muscle development and postnatal function.
-
The human CAV3 gene is mapped on chromosome 3p25 and is organized in two coding exons.
-
Most of caveolinopathies are transmitted with an autosomal dominant inheritance, and only six autosomal recessive CAV3 mutations have been described.
-
Caveolin-3 deficiency leads to four skeletal muscle phenotypes: Limb Girdle Muscular Dystrophy (LGMD) 1C, Isolated HyperCKemia, Rippling Muscle Disease, Distal Myopathy. CAV3 mutations were reported also in one case of Familial Hypertrophic Cardiomyopathy, 4 patients affected by Long QT Syndrome and 3 infants died from Sudden Infant Death Syndrome.
-
Many patients show such an overlap of the four skeletal muscle phenotypes above described that few authors suggest that caveolinopathies should be considered as "a clinical continuum".
-
In caveolinopathies genotype-phenotype correlations do not exist, as studies have shown that the same mutation lead to heterogeneous clinical diseases.
-
In LGMD-1c patients, histological analysis may reveal variably sized, degenerating muscle fibers, with an increased number of central nuclei and a mild substitution of connective tissue.
-
Patients need symptomatic care aimed to preserve muscle function and treat complications particularly the ones with LGMD. Periodic monitoring of spine, respiratory and cardiac function, must be provided according to each patient individual needs.
Introduction
Caveolae were initially discovered by electron microscopy and described as flask-shaped invaginations of the plasma membrane. Caveolar architecture is formed by caveolins, a family of proteins composed of three isoforms, caveolin-1 (Cav-1), -2 and -3.1, 2, 3 All the three genes encoding each family member are evolutionarily conserved and Cav-1 and Cav-3 amino acid sequences display a high degree of homology.
Cav-1 is expressed in a variety of tissues, particularly in terminally differentiated cells, such as adipocytes, endothelia, smooth muscle cells, and type I pneumocytes. Cav-2 is usually co-expressed and forms hetero-oligomers with Cav-1, whereas Cav-3 has been described in skeletal and smooth muscle tissue and also, more recently, in glial cells and early post-natal peripheral nerves.1, 2, 3, 4
The critical role of Cav-3 in muscle cell physiology was shown first by in vitro and in vivo studies and was conclusively confirmed by the findings that mutations in caveolin-3 gene (CAV3) lead to distinct neuromuscular and cardiac disorders such as Limb Girdle Muscular Dystrophy (LGMD) 1-C, idiopathic persistent elevation of serum creatine kinase (H-CK), inherited rippling muscle disease (RMD), distal myopathy (MD) and familial hypertrophic cardiomyopathy (HCM).1, 5, 6 Notably, over the past few years, mutations in the CAV3 gene have been found in the arrhythmogenic long QT syndrome (LQTS) and in sudden infant death syndrome (SIDS).
Caveolin-3 in muscle development and physiology
Cav-3 protein is 151 amino acids (aa) long and is divided in five separate domains: N-terminal (aa 1–53), scaffolding (aa 54–73), transmembrane (aa 74–106), and C-terminal (aa 107–151). The N-terminal domain contains a signature sequence (aa 41–48, FEDVIAEP) that is present in all caveolins.2 The scaffolding segment is responsible for Cav-3 homo-oligomerization, a process which starts in the endoplasmic reticulum and preludes to the organization of detergent-resistant caveolar complexes of approximately 25–50 nm in diameter. These structures hence fuse with the plasma membrane forming the final caveolae (Figure 1). The transmembrane domain forms a hairpin loop in the sarcolemma, allowing both the N- and C-terminal ends to face the cytoplasm.5
Cav-3 is synthesized in the endoplasmic reticulum (ER) and is then transported to the Golgi complex as detergent-soluble oligomers. When Cav-3 exits the Golgi complex, the oligomers associate with glycosphingolipid and cholesterol-enriched lipid-raft domains, as judged by detergent-resistant membrane (DRM) association, and forms the caveolar carriers. These complexes finally fuse with the plasma membrane defining the caveolae invaginations.
In adult myofibers, Cav-3 is present throughout the T tubule system, where it is clustered in so called ‘hot spots’ at the necks of the tubules in the subsarcolemmal space. In these areas, localized just inside the opening of the T tubules and therefore in sites critical for the electric transmission of the contractile impulse, caveolae function as platforms which concentrate on ion channels, kinases, and signaling molecules.7
Within the sarcolemma, Cav-3 belongs to the dystrophin–glycoprotein complex (DGC), which provides a link between the cytoskeleton and the extracellular matrix, and is essential to confer stability to the muscle cell membrane.8, 9 Cav-3 and dystrophin competitively bind to the same site of β-dystroglycan and changes in Cav-3 expression as in Cav-3 transgenics lead to the disruption of the DGC and to downregulation of dystrophin protein levels.10, 11, 12, 13
Muscle caveolae are implied also in the regulation of different signaling pathways. Indeed, signaling molecules such as Gi2α, Gβγ, c-Src, Src kinases, GPI-linked proteins, nitric oxide synthases (neuronal and inducible NOS) and type I myostatin receptors display a specific caveolin-binding domain motif which interacts with Cav-3 scaffolding segment.14, 15, 16, 17 In cardiomyocites, the ion channels HCN4, Cav1.2, Kv1.5, kir6.2/Sur2a, Nav1.5, and NCX are targeted to caveolae, and Cav-3 directly interacts with β2-adrenoreceptors with whom it regulates the sarcolemmal targeting of cyclic AMP signal.18, 19, 20, 21, 22 Notably, chemical disruption of caveolae affects the excitation–contraction coupling and β-adrenergic responsiveness of adult cardiac myocites.21, 22
Likewise, Cav-3 has a role in the regulation of energy metabolism of muscle cells. Indeed, Cav-3 regulates the expression of the insulin receptor on muscle membrane, it modulates the translocation upon insulin induction of the glucose transporter 4, and is required for the cell membrane targeting of phosphofructokinase (PFK), an enzyme that catalyzes a rate-limiting reaction in glycolysis.23, 24, 25 Finally, caveolae facilitates free fatty cellular uptake by interacting with several fatty acid transport proteins.26
In vitro Cav-3 functional characterization determined that this molecule regulates myoblast cell differentiation and survival.
During the differentiation of skeletal myoblasts, Cav-3 is increased by the coordinated activity of p38 kinase and the PI3 kinase-AKT-mTor-signaling pathways.8, 27 Cav-3 antisense inhibition precludes myoblast fusion and myotube formation, normal processes of skeletal muscle development.28 Consistently, stably overexpression of the Cav-3 mutant P104L in C2C12 cells results into persistence of a severe undifferentiated phenotype associated to a decrease in the expression of muscle differentiation markers and to an increase of the muscle-specific ubiquitin ligase Atrogin-1.29
The role of Cav-3 in muscle development and physiology was confirmed by in vivo studies.
Cav-3 activities in muscle tissue differentiation and specification were investigated in zebrafish, a readily accessible model for muscle disease. These experiments showed that Cav-3 is expressed early in development within the notochord and Cav-3 gene deletion results into notochord defects. Embryos injected with Cav-3 morpholino antisense oligonucleotides display decreased and disorganized myoblast fusion. In myotube precursors, the contractile apparatus is characterized by chaotic filament bundles with interspersed many mitochondria and a poorly developed T-tubule network. This phenotype results into embryos with slowed or completely uncoordinated movements.30
Preservation of physiological Cav-3 levels is essential also for normal post-natal skeletal muscle function.
Cav-3 knockout mice lack muscle cell caveolae and display moderate signs of muscular dystrophy. In this model, targeted cav-3 gene deletion leads to defects of the T-tubule system.31
Notably, also transgenic mice overexpressing wild-type Cav-3 display degeneration and necrosis of muscle fibers and an increase of serum creatine kinase (CK) levels. These skeletal muscle defects resemble the phenotype observed in Duchenne Muscular Dystrophy. This is not surprising because at the histological examination, Cav-3 overexpression leads to downregulation of dystrophin and ß-dystroglycan expression.13 It is possible that high Cav-3 levels could sequester β-dystroglycan thus disrupting the assembly of the DGC and addressing dystrophin to proteolytic degradation.
These results were corroborated and extended by Sunada et al32 who generated and characterized transgenic mice overexpressing the Cav-3 P104L mutant. This model shows severe skeletal muscle atrophy associated with a significant increase in neuronal nitric oxide synthase and to an over-activation of myostatin-signaling pathway.
Myostatin, a member of the transforming growth factor-β superfamily is a main player in the negative regulation of skeletal muscle volume. Myostatin overexpression causes severe muscle atrophy, whereas targeted disruption markedly increase muscle mass in mice.33, 34
The generation of Cav-3 mutated animals indicated a possible role of CAV3 mutations also in the pathogenesis of cardiac disorders.
Cav-3 is expressed in cardiomyocites where it colocalizes with different molecules involved in the development of myocardium hypertrophy, such as heterotrimeric G proteins, protein kinase C (PKC), Ras, extracellular signal-regulated protein kinase and cNOS. As Cav-3 inhibits the activation of these signaling pathways, it could be hypothesized that caveolae might function as a control center of cellular signaling of cardiomyocite hypertrophy.35 Several are the in vivo data which support this hypothesis.
In rats, adenovirus-driven overexpression of Cav-3 inhibits agonist-induced cardiomyocite hypertrophy, whereas the dominant-negative Cav-3 enhances this process.36
Interestingly, transgenic mice overexpressing the Cav-3 wild type or Cav-3 P104L mutant develop unique pathophysiological characteristics of HCM such as increased thickness of the interventricular septum and left ventricular posterior wall, hypercontractility, and diastolic dysfunction.37
In accordance, Cav-3 knockout mice display myocardium hypertrophy, dilation, and reduced fractional shortening by 4 months of age. Histologically, the cardiac muscle shows signs of inflammation such as cellular infiltration associated to perivascular fibrosis. Microscopically the cardiomyocites are hypertrophic and display a reduced expression of the DGC complex. This phenotype is enhanced in Cav-1/Cav-3 double knockout mice, which completely lack morphologically identifiable caveolae. This mouse model shows a significant increase in the thickness of the left ventricle wall associated to an upregulation of atrial natriuretic factor. Microscopically, the cardiac muscle is disorganized with signs of inflammation and perivascular fibrosis. The cardiac myocites also show severe signs of hypertrophy and degeneration.38
In this context, it is important to underline that Cav-3 null animals also show increased adiposity and development of insulin resistance, thus suggesting that Cav-3 might be involved in the regulation of whole body glucose homeostasis.39, 40
Skeletal muscle diseases associated to caveolin-3 deficiency
The human CAV3 gene is mapped on chromosome 3p25 and is organized in two coding exons.41 Several specific CAV3 mutations leading to a type of skeletal muscular disease have been identified. Specifically at present, 24 different missense mutations, 1-bp insertion, 3 few base pair deletions, 1 genomic macrodeletion and 1 splice site substitution have been reported (Tables 1, 2, 3, 4).
Most of the caveolinopathies are inherited with an autosomal-dominant inheritance, and only six autosomal recessive CAV3 mutations have been described. McNally et al42 reported a p.G55S missense substitution causing LGMD-1C. Kubisch et al43 described a homozygous p.L85P exchange in one patient affected by RMD and displaying complete loss of Cav-3 in the muscle biopsy. Unfortunately, clinical and genetic analysis of additional family members were not performed. Muller et al44 reported a patient exhibiting a homozygous splice-site CAV3 mutation resulting in aberrant splicing, in-frame deletion of 14 amino acids, and loss of Cav-3 protein in the muscle biopsy. The clinical phenotype was compatible with mild LGMD. Sequence analysis and family history indicated an autosomal recessive inheritance. Ueyama et al45 reported a 39-year-old patient with RMD who carried a homozygous CAV3 change (p.W70X). The patient also had extraocular muscle paresis showing atrophy of the extraocular muscles on orbital MRI. Traverso et al46 described autosomal recessive RMD due to a compound heterozygosity, CAV3 exon 2 macrodeletion/1-bp insertion in exon 1. Finally, the same group identified one patient affected by dilated cardiomyopathy and LGMD-1C and carrying a Cav-3 p.T77M substitution.47
In a screening of 663 patients affected by primary myopathies of unknown etiology, CAV3 mutations represented the one percent of unclassified LGMD and other phenotypes including isolated H-CK, RMD and proximal and distal myopathy.48 In addition, four CAV3 mutants have been identified in patients affected by LQTS and SIDS, confirming a role for Cav-3 in the regulation of cardiac ion channels.49, 50
Aboumousa et al recently analyzed a cohort of 10 UK patients from 6 families with genetically confirmed CAV3 mutations. Muscle pain was the main presenting symptom, whereas muscle weakness was variable and did not correlate with the duration of the disease. Manifestations of muscle hyperirritability (rippling muscle movements and percussion-induced rapid contractions – PIRCS) were highly suggestive of CAV3 mutations, whereas serum CK levels were inconstantly increased and did not correlate with disease progression. Interestingly, one patient displayed episodes of myoglobinuria and another experienced episodes of hypoglycemia. The authors emphasize that the mislocalization of the glycolitic enzyme PFK observed in experimental models of Cav-3 deficiency could provide a molecular rationale to the myalgias and the myoglobinuria verified in their group of patients. Indeed, the same signs are present in glycogenosis type VII (PFK congenital deficiency). Similarly, the episodes of hypoglycemia described in one patient would agree with the insulin resistance determined in Cav-3 knockout animals. Hence, this study strongly suggests a proper evaluation of glucose homeostasis in caveolinopathic patients.51
Limb girdle muscular dystrophy-1C
The term LGMD describes childhood- or adult-onset muscular dystrophies characterized by weakness and wasting restricted to the limb musculature, proximal greater than distal. Skeletal muscle biopsy typically shows degeneration/regeneration of muscle cells, associated with elevated serum CK levels. Immunostaining performed on muscle biopsy and genetic analysis can define the diagnosis of the LGMD subtypes.52
In a recent analysis conducted in a large sample of genetically diagnosed LGMD Italian patients, CAV3 mutations represented the 5% of the probands.53
The first description of an LGMD associated with Cav-3 deficiency was by Minetti et al54 who reported affected individuals in two unrelated Italian families. The disease had onset of symptoms at approximately age five, and mild-to-moderate proximal muscle weakness, calf pseudohypertrophy, positive Gower sign, and serum CK concentrations approximately 4-fold to 25-fold higher than normal were observed. Two individuals from the same family had muscle cramps after exercise.
Subsequent reports underlined the variability of LGMD-1C clinical features and included:
(1) a 4-year-old German girl with myalgias in the lower limbs but no muscle weakness; (2) a 4-year-old girl with myalgias and a dystrophic pattern in the skeletal muscle biopsy; (3) an 11-year-old Japanese girl with a history of floppiness at birth, marginally delayed motor milestones, progressive proximal muscle weakness, and exercise-induced myalgias; (3) a 71-year-old woman, without any previous neuromuscular symptoms, who had mild proximal muscle weakness, scapular winging, slight calf hypertrophy, and a positive Gower sign; (4) two Japanese boys (3- and 6-years old) who displayed muscle pain, calf hypertrophy, increased CK levels, but no muscle weakness; (5) one 57-year-old woman with mild proximal weakness of the lower limbs, calf pseudohypertrophy, high CK levels; (6) a 58-year-old woman with myalgias, proximal weakness, calf pseudohypertrophy and dilatative cardiomyopathy (Table 1).12, 44, 55, 56, 57, 58
In LGMD-1C patients, electromyographic studies range from a normal to a myopathic pattern, whereas muscle biopsy analysis can show variably sized, degenerating/regenerating muscle fibers, with an increased number of central nuclei, and a mild substitution of connective tissue. The expression of Cav-3 is reduced invariably both by immunohistochemistry and immunoblot analysis in muscle fibers. This disorder is characterized by a benign clinical course, there is no evidence of respiratory impairment and life expectancy is not reduced.59
Notably, few CAV3 mutations are associated with LGMD-1C and different clinical phenotypes in the same family, and few patients display overlapping signs between LGMD and RMD.43, 60, 61
HyperCKemia
Isolated H-CK, that is, increased serum concentration of CK in the absence of any clinical findings of muscular disease can occur in simplex (that is, a single occurrence in a family) or familial cases.
Sporadic H-CK
Soon after the initial identification of CAV3 mutations linked to LGMD-1C, a novel sporadic CAV3 p.R26Q mutant was identified in two unrelated 4- and 6-year-old children. Unlike patients with LGMD-1C, these children only had persistent H-CK (serum CK approximately 4 to 8 higher than normal). Histologic muscle analysis revealed only mild fiber size variability in one child. No other myopathic features were evident.62, 63 Similar clinical features were reported in H-CK (s) patients identified in later studies (Table 2).
Familial H-CK
The CAV3 p.P28L substitution was identified in a mother and her son affected by H-CK (CK ∼4- to 17-fold greater than normal). There was no history of muscle symptoms nor were there any findings on routine physical muscle examination. Histologic muscle analysis showed high muscle fiber size variability, hypertrophic fibers, a few internal nuclei, and one necrotic fiber.64
Subsequent reports of familial H-CK due to Cav-3 deficiency were described in three members of a Spanish family who displayed persistent elevated serum CK concentrations (CK from 3 to 10 higher than normal) without any muscle weakness. Calf pseudohypertrophy was present in the proband.65
Rippling muscle disease
RMD is characterized by signs of increased muscle irritability, such as percussion-induced rapid contraction (PIRC), percussion-induced muscle mounding (PIMM), and/or electrically silent muscle contractions (rippling muscle).
Betz et al66, 67 identified CAV3 mutations in five previously described families of autosomal-dominant RMD. In one of the families, five individuals from three generations had late childhood or early teen onset of proximal muscle stiffness and PIMM. All had muscle hypertrophy. Seven other relatives had PIMM, but apparently no other muscle symptoms. Muscle biopsy showed increases in fiber size variability, centralized nuclei, and mild type-1 fiber predominance. Subsequent clinical descriptions indicate that the age of onset of disease is variable (3–36 years), whereas the presenting symptoms are usually, fatigue, tiptoe walking, myalgias, and muscle stiffness. Calf pseudohypertrophy can be detected. Muscle hypertony, PIRCs, PIMMs and a rippling phenomenon are represented differently in the group of the patients (Table 3).43, 45, 46, 67, 68, 69, 70, 71, 72, 73, 74
Distal myopathy (DM)
There are only two reports of distal myopathy due to CAV3 mutations. Interestingly both cases are associated with the same Cav-3 aa change p.R26Q.
One 25-year-old Japanese woman was found with muscle atrophy in her hands and feet and decreased distal muscle function, with normal proximal muscle strength. Her serum CK concentration was 25 times increased. Histological analysis of her biceps brachii showed mild variation in fiber size, an increased number of centralized nuclei, and a predominance of type 1 fibers. Cav-3 was markedly reduced.75
The second description refers to a Spanish family whose members developed an autosomal-dominant hand-involved distal myopathy. Interestingly, the youngest members displayed signs of muscle hyperexcitability and H-CK before the distal myopathy became apparent clinically. Muscle biopsies revealed no dystrophic changes, but slight variation in fiber size and increased number of internal nuclei. Cav-3 expression was greatly decreased.76
Familial hypertrophic cardiomyopathy
Although both Cav-3 knockout and transgenic mouse models show reproducible signs of heart involvement with hypertrophic cardiomyopathy progressing to diastolic dysfunction, in humans the vast majority of CAV3 mutations do not cause cardiac phenotypes and the two single reports available in the literature seem to implicate different roles of Cav-3 in the skeletal and cardiac muscle tissues.
Hayashi et al77 described two siblings affected by HCM associated with a CAV3 p.T63S substitution. Neither had any skeletal muscle manifestations; serum CK concentrations were normal.
On the other hand, Cav-3 expression and caveolar structures were normal in the cardiac muscle tissue of a patient affected by a CAV3 p.F96 deletion leading in his family to LGMD-1C, H-CK and RMD and to a severe Cav-3 deficit in skeletal muscle.78
Only recently, Traverso et al reported a 58-year old patient affected by LGMD-1C and dilatative cardiomyopathy (p.T77M mutation).47
It is possible that compensatory mechanisms to Cav-3 deficiency are different in cardiac and skeletal muscle tissues, but the molecular basis for this difference is not known.
Extensive clinical examination of cardiac function in LGMD-1C, RMD and H-CK Cav-3 patients and mutational screening of large populations will be necessary to assess the role of CAV3 mutations in heart disease.
Overlapping muscle diseases and genotype–phenotype correlations in caveolinopathies
Mutations in the CAV3 gene can cause a wide spectrum of clinical phenotypes. Many patients show such an overlap of the four skeletal muscular diseases above described that few authors suggest that caveolinopathies should be considered as a ‘clinical continuum’.43
In this group of diseases, moreover, genotype–phenotype correlations do not exist, as studies have shown that the same mutation can lead to heterogeneous clinical phenotypes and muscle histopathological changes.48 For example, the CAV3 mutation p.T63S found in a case of HCM is analogous to mutations [p.T63P and p.TFT (63–65)] identified in association with LGMD-1C.54, 77
Similarly, the same p.R26Q CAV3 mutation was reported in a family with extreme phenotypic variability. Of the 11 individuals with the confirmed mutation, three exhibited both RMD and LGMD-1C features, two had predominantly LGMD1C characteristics, two, both minors, had muscle stiffness only, and one was asymptomatic.62
Likewise, Fischer et al61 described family members with different, but overlapping, muscle disease phenotypes. RMD was evident in all 14 affected individuals. Among these, 12 also had evidence of LGMD -1 or DM.
In two Japanese families four of the six individuals with RMD also had DM, whereas in an Italian family, patients showed the LGMD-1C, RMD, and H-CK phenotypes.62, 79
Management of caveolinopathic patients
Symptomatic supportive care aimed to preserve muscle function, maximize functional ability and treat complications is particularly important in those patients with LGMD. Weight control to avoid obesity, physical therapy and stretching exercises to promote mobility and prevent contractures, use of mechanical aids and social and emotional support must be provided. The prevention of secondary complications includes special precautions during surgical procedures and anesthesia because of possible risk for malignant hyperthermia in individuals with H-CK. The periodic monitoring of spine, respiratory function, cardiac function, mobility, and muscle function must be provided based on individual needs.
Caveolin-3 and heart hereditary arrhythmogenic disorders
Over the past few years, the interest on Cav-3 regulation on the intracellular trafficking and posttranslational modifications of different cardiac ion channels and β2-adrenoreceptors has been constantly growing.
Accordingly, 4CAV3 mutations were identified in 905 patients referred for LQTS (Table 4). Electrophysiological studies revealed that at least two of these Cav-3 mutants altered the function of the ion channel Nav1.5.49 These patients did not exhibit signs of skeletal muscle involvement or primary cardiomyopathy, thus implicating a possible role for Cav-3 and caveolae in cardiac excitability. (Table 4)
These results were further developed by Cronck et al50 who identified three CAV3 mutations (V14L, T78M, L79R) in a population of 133 infants died from SIDS. Voltage clamp analysis revealed that all three Cav-3 mutants caused a fivefold increase in late sodium current. (Table 4)
Pathogenetic mechanisms of muscle tissue damage in Caveolin-3 deficiency
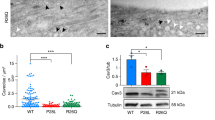
Most of CAV3 mutations cause a severe loss of Cav-3 protein as shown both by immunohistochemistry and immunoblot analysis on muscle biopsies (Figures 2a and b). Only one report described two cases of H-CK and proven CAV3 mutations (p.G55S and p.T77M) with normal Cav-3 expression at the muscle biopsy. It is feasible that these mutants result into a stable but dysfunctional protein.63
CAV3 mutations cause a severe decrease in Cav-3 expression and membrane localization. (a) Frozen sections of the skeletal muscle biopsy from one representative patient (Pt) and an age-matched control (C) were prepared and immunostained with specific antibodies against caveolin-3 (Cav-3). In control muscle Cav-3 displays a uniform pattern at the sarcolemma. In the patient, Cav-3 is markedly reduced. Final magnification, × 40. (b) Protein lysates prepared from the skeletal muscle biopsy from one patient (Pt) and an age-matched control (C) were separated by SDS-PAGE and subjected to western immunoblot analysis using antibodies against Cav-3. In the patient Cav-3 protein levels are reduced by approximately 80%. Modified and reproduced by Traverso et al.47
The molecular mechanisms implied in the pathogenesis of the muscle degenerative process in Cav-3 deficiency are multiple and only partially clarified.
As a first, caveolae are essential in the development and maintenance of skeletal muscle membrane architecture. Indeed, electron microscopy analysis of muscle samples from patients with LGMD-1C showed that loss of caveolae at the sarcolemma was associated with the formation of large subsarcolemmal membranous vacuoles and ‘honeycomb’ membranous structures seen during abnormal proliferation of the T-tubule system.7 These ultrastructural changes were further confirmed in subsequent reports, including patients affected by DM or RMD.5, 59
Secondarily, functional characterization of few CAV3 mutations (p.P104L, p. ΔTFT 63–65 p.R26Q and p.T77K) indicated that these mutants form unstable high molecular mass aggregates that are retained in the Golgi complex and are not correctly targeted to the plasma membrane. Consistently with their autosomal-dominant inheritance, these mutations can cause retention of wild-type Cav-3 in the Golgi compartment thus inducing the proteolysis of wild-type Cav-3 by ubiquitination and proteasomal degradation (Figures 3 and 4).80, 81 Accordingly, stable overexpression of Cav-3 P104L in C2C12 cells induces a significant upregulation of the ubiquitin ligase atrogin-1.29
Cav-3 mutants are retained in an intracellular compartment and are expressed at lower levels. Cos-7 cells were transiently transfected with cDNA encoding either for Cav-3 wild type (WT) or the Cav-3 mutant T78K. (a) Thirty-six hours post-transfection, cells were fixed and stained with antibodies against Cav-3. Cav-3 WT is localized at the plasma membrane, as expected, whereas Cav-3 T78K is retained in an intracellular, perinuclear compartment. N, nucleus. Final magnification, × 100. (b) Thirty-six hours post-transfection, cells were extracted and protein lysates subjected to western immunoblot analysis with antibodies against Cav-3. Cav-3 T78K is expressed at much lower levels than Cav-3 WT. Modified and reproduced by Traverso et al.46
Cav-3 mutants cause the intracellular retention of Cav-3 wild-type form. Cos-7 cells were transiently co-transfected with cDNA encoding GFP-Cav-3 wild type (WT) in combination either with Cav-3 WT or the Cav-3 T78K mutant. Thirty-six hours post-transfection, cells were fixed and stained with antibodies against GFP. When co-transfected with Cav-3 WT or GFP-Cav-3 WT is localized at the plasma membrane, as expected. When co-transfected with Cav-3 T78K, GFP-Cav-3 WT is retained in a perinuclear compartment, suggesting that Cav-3 T78K behaves in a dominant-negative manner. N, nucleus. Final magnification, × 100. Modified and reproduced by Traverso et al.46
In a complementary set of experiments, Smythe et al82 determined that the p.ΔTFT 63-65 Cav-3 mutant in post-mitotic skeletal myotubes severely reduces the binding of the signal molecule Src to Cav-3, diminishes targeting of Src to lipid rafts, and causes abnormal perinuclear accumulation of Src. Along with these alterations of Src localization and targeting, Src activation is elevated in myotubes expressing this mutation and an increased incidence of apoptosis in those cells compared with control myotubes is observed. These results indicate that CAV3 mutations disrupt normal cellular signal transduction pathways, alter muscle cell structural integrity, and cause apoptosis.
On the other side, Sunada et al83 showed that in vivo overexpression of the Cav-3 P104L mutant leads to an over-activation of myostatin-signaling pathway, as evidenced by the increased phosphorylation of the myostatin downstream effectors Smad2/3. Indeed, Cav-3 P104L transgenic mice when crossed with mice overexpressing the myostatin prodomain, an inhibitor of myostatin activation, display a rescue of the functional (muscle weakness) and histopathological signs of muscle atrophy. Similar results were obtained when the animals were injected with a soluble ActRIIB-FC fusion protein, which inhibits myostatin receptor binding.
Finally, it is important to underline that patients with LGMD-1C and experimental models of p.P104L and p.ΔTFT63-65 CAV3 mutations also manifest mislocalization of dysferlin, a muscle membrane protein which is decreased in Miyoshi myopathy and LGMD-2B.56 In physiological conditions, dysferlin interacts with Cav-3 on the muscle sarcolemmma, whereas in Cav-3 deficiency it accumulates in the cytoplasm or it displays an irregular ‘patchy’ distribution on the membrane.56 Indeed, Cav-3 modulates dysferlin sarcolemmal levels by inhibiting its endocytosis through a clathrin-independent pathway.84
Analysis of muscle biopsies from patients affected by Myoshi myopathy indicates that dysferlin deficiency leads to severe disruptions of the structure of the sarcolemma thus suggesting an important role for dysferlin in muscle cell structure.85 It is therefore possible that changes in dysferlin cellular localization may contribute to the pathogenesis of Cav-3-associated disorders.
Conclusions
Over the past years, several clinical reports have followed and greatly extended the first identification of CAV3 mutations in patients affected by LGMD-1C, and multiple basic studies have shed light on caveolae functions in skeletal muscle. According to us, few issues should be emphasized.
-
1
In caveolinopathies, genotype–phenotype correlations cannot be traced and a relevant intrafamilial phenotypic variability has been described. Additional genetic and/or environmental modifiers are involved and have not been identified yet.
-
2
The characterization of Cav-3 genetically modified animal models have provided several hints to the analysis of the pathogenetic mechanisms underlying the process of muscle degeneration in Cav-3 deficiencies. Although these models suggest a precise role for Cav-3 defects in the genesis of hypertrophic cardiomyopathy, the data in humans are extremely limited and controversial.
-
3
Cav-3 knockout models and in vitro data indicate a role for Cav-3 in the regulation of muscle and whole body glucose homeostasis. These observations could represent the molecular rationale for few of the muscular and systemic signs/symptoms observed in the patients and, therefore, should be further developed in the clinical setting.
-
4
Cav-3 regulation of cardiac ion channels and its involvement in LQTS is an exciting chapter and is suggestive of future pathophysiological studies.
-
5
Up to now, only symptomatic supportive treatment is available for caveolinopathic patients. Recently, administration of a cell permeable peptide containing the homeodomain of the antennapedia (penetratin) coupled to Cav-1 scaffolding domain was able to decrease tumor growth in two neoplastic mouse models, and to decrease pulmonary hypertension (PH) in a monocrotaline-induced PH rat model.86, 87, 88, 89 Studies aimed to explore the possible effects of a Cav-3 scaffolding peptide have not yet been developed. Moreover, the phenotype improvement observed in Cav-3 P104L transgenic animals following systemic injection with a myostatin inhibitor might represent a novel strategy in the treatment of caveolinopathies.83
References
Williams TM, Lisanti MP : The Caveolin genes: from cell biology to medicine. Ann Med 2004; 36: 584–595.
Parton RG : Caveolae and caveolins. Curr Opin Cell Biol 1996; 8: 542–548.
Scherer PE, Okamoto T, Chun M, Nishimoto I, Lodish HF, Lisanti MP : Identification, sequence, and expression of caveolin-2 defines a caveolin gene family. Proc Natl Acad Sci USA 1996; 93: 131–135.
Lee HJ, Park CH, Lee SJ et al: Expression of caveolin-3 immunoreactivities in the developing sciatic nerve of the rat. Muscle Nerve 2008; 38: 1021–1026.
Galbiati F, Razani B, Lisanti MP : Caveolae and caveolin-3 in muscular dystrophy. Trends Mol Med 2001; 7: 435–441.
Woodman SE, Sotgia F, Galbiati F, Minetti C, Lisanti MP : Caveolinopathies: mutations in caveolin-3 cause four distinct autosomal dominant muscle diseases. Neurology. 2004; 62: 538–543.
Minetti C, Bado M, Broda P et al: Impairment of caveolae formation and T-system disorganization in human muscular dystrophy with caveolin-3 deficiency. Am J Pathol 2002; 160: 265–270.
Song KS, Scherer PE, Tang Z et al: Expression of caveolin-3 in skeletal, cardiac, and smooth muscle cells. Caveolin-3 is a component of the sarcolemma and co-fractionates with dystrophin and dystrophin-associated glycoproteins. J Biol Chem 1996; 271: 15160–15165.
Crosbie RH, Yamada H, Venzke DP, Lisanti MP, Campbell KP : Caveolin-3 is not an integral component of the dystrophin glycoprotein complex. FEBS Lett 1998; 427: 279–282.
Ilsley JL, Sudol M, Winder SJ : The WW domain: linking cell signalling to the membrane cytoskeleton. Cell Signal 2002; 14: 183–189.
Sotgia F, Lee JK, Das K et al: Caveolin-3 directly interacts with the C-terminal tail of beta -dystroglycan. Identification of a central WW-like domain within caveolin family members. J Biol Chem 2000; 275: 38048–38058.
Herrmann R, Straub V, Blank M et al: Dissociation of the dystroglycan complex in caveolin-3-deficient limb girdle muscular dystrophy. Hum Mol Genet 2000; 9: 2335–2340.
Galbiati F, Volonte D, Chu JB et al: Transgenic overexpression of caveolin-3 in skeletal muscle fibers induces a Duchenne-like muscular dystrophy phenotype. Proc Natl Acad Sci USA 2000; 97: 9689–9694.
Venema VJ, Ju H, Zou R, Venema RC : Interaction of neuronal nitric-oxide synthase with caveolin-3 in skeletal muscle. Identification of a novel caveolin scaffolding/inhibitory domain. J Biol Chem 1997; 272: 28187–28190.
Shaul PW, Anderson RG : Role of plasmalemmal caveolae in signal transduction. Am J Physiol 1998; 275: L843–L851.
Zajchowski LD, Robbins SM : Lipid rafts and little caves. Compartmentalized signalling in membrane microdomains. Eur J Biochem 2002; 269: 737–752.
Sotgia F, Razani B, Bonuccelli G et al: Intracellular retention of glycosylphosphatidyl inositol-linked proteins in caveolin-deficient cells. Mol Cell Biol 2002; 22: 3905–3926.
Yarbrough TL, Lu T, Lee HC, Shibata EF : Localization of cardiac sodium channels in caveolin-rich membrane domains: regulation of sodium current amplitude. Circ Res 2002; 90: 443–449.
Martens JR, Sakamoto N, Sullivan SA, Grobaski TD, Tamkun MM : Isoform-specific localization of voltage-gated K+ channels to distinct lipid raft populations. Targeting of Kv1.5 to caveolae. J Biol Chem 2001; 276: 8409–8414.
Bossuyt J, Taylor BE, James-Kracke M, Hale CC : The cardiac sodium-calcium exchanger associates with caveolin-3. Ann NY Acad Sci 2002; 976: 197–204.
Balijepalli RC, Foell JD, Hall DD, Hell JW, Kamp TJ : Localization of cardiac L-type Ca(2+) channels to a caveolar macromolecular signaling complex is required for beta(2)-adrenergic regulation. Proc Natl Acad Sci USA 2006; 103: 7500–7505.
Barbuti A, Terragni B, Brioschi C, DiFrancesco D : Localization of f-channels to caveolae mediates specific beta2-adrenergic receptor modulation of rate in sinoatrial myocytes. J Mol Cell Cardiol 2007; 42: 71–78.
Sotgia F, Bonuccelli G, Minetti C et al: Phosphofructokinase muscle-specific isoform requires caveolin-3 expression for plasma membrane recruitment and caveolar targeting: implications for the pathogenesis of caveolin-related muscle diseases. Am J Pathol 2003; 163: 2619–2634.
Karlsson M, Thorn H, Parpal S, Stralfors P, Gustavsson J : Insulin induces translocation of glucose transporter GLUT4 to plasma membrane caveolae in adipocytes. FASEB J 2002; 16: 249–251.
Scherer PE, Lisanti MP : Association of phosphofructokinase-M with caveolin-3 in differentiated skeletal myotubes. Dynamic regulation by extracellular glucose and intracellular metabolites. J Biol Chem 1997; 272: 20698–20705.
Augustus AS, Buchanan J, Addya S et al: Substrate uptake and metabolism are preserved in hypertrophic caveolin-3 knockout hearts. Am J Physiol Heart Circ Physiol 2008; 295: H657–H666.
Fanzani A, Musaro A, Stoppani E et al: Hypertrophy and atrophy inversely regulate Caveolin-3 expression in myoblasts. Biochem Biophys Res Commun 2007; 357: 314–318.
Galbiati F, Volonte D, Engelman JA, Scherer PE, Lisanti MP : Targeted down-regulation of caveolin-3 is sufficient to inhibit myotube formation in differentiating C2C12 myoblasts. Transient activation of p38 mitogen-activated protein kinase is required for induction of caveolin-3 expression and subsequent myotube formation. J Biol Chem 1999; 274: 30315–30321.
Fanzani A, Stoppani E, Gualandi L et al: Phenotypic behavior of C2C12 myoblasts upon expression of the dystrophy-related caveolin-3 P104 L and TFT mutants. FEBS Lett 2007; 581: 5099–5104.
Nixon SJ, Wegner J, Ferguson C et al: Zebrafish as a model for caveolin-associated muscle disease; caveolin-3 is required for myofibril organization and muscle cell patterning. Hum Mol Genet 2005; 14: 1727–1743.
Galbiati F, Engelman JA, Volonte D et al: Caveolin-3 null mice show a loss of caveolae, changes in the microdomain distribution of the dystrophin-glycoprotein complex, and t-tubule abnormalities. J Biol Chem 2001; 276: 21425–21433.
Sunada Y, Ohi H, Hase A et al: Transgenic mice expressing mutant caveolin-3 show severe myopathy associated with increased nNOS activity. Hum Mol Genet 2001; 10: 173–178.
McPherron AC, Lawler AM, Lee SJ : Regulation of skeletal muscle mass in mice by a new TGF-beta superfamily member. Nature 1997; 387: 83–90.
Grobet L, Pirottin D, Farnir F et al: Modulating skeletal muscle mass by postnatal, muscle-specific inactivation of the myostatin gene. Genesis 2003; 35: 227–238.
Kikuchi T, Oka N, Koga A, Miyazaki H, Ohmura H, Imaizumi T : Behavior of caveolae and caveolin-3 during the development of myocyte hypertrophy. J Cardiovasc Pharmacol 2005; 45: 204–210.
Koga A, Oka N, Kikuchi T, Miyazaki H, Kato S, Imaizumi T : Adenovirus-mediated overexpression of caveolin-3 inhibits rat cardiomyocyte hypertrophy. Hypertension 2003; 42: 213–219.
Ohsawa Y, Toko H, Katsura M et al: Overexpression of P104L mutant caveolin-3 in mice develops hypertrophic cardiomyopathy with enhanced contractility in association with increased endothelial nitric oxide synthase activity. Hum Mol Genet 2004; 13: 151–157.
Woodman SE, Park DS, Cohen AW et al: Caveolin-3 knock-out mice develop a progressive cardiomyopathy and show hyperactivation of the p42/44 MAPK cascade. J Biol Chem 2002; 277: 38988–38997.
Capozza F, Combs TP, Cohen AW et al: Caveolin-3 knockout mice show increased adiposity and whole body insulin resistance, with ligand-induced insulin receptor instability in skeletal muscle. Am J Physiol Cell Physiol 2005; 288: C1317–C1331.
Cohen AW, Razani B, Wang XB et al: Caveolin-1-deficient mice show insulin resistance and defective insulin receptor protein expression in adipose tissue. Am J Physiol Cell Physiol 2003; 285: C222–C235.
Sotgia F, Minetti C, Lisanti MP : Localization of the human caveolin-3 gene to the D3S18/D3S4163/D3S4539 locus (3p25), in close proximity to the human oxytocin receptor gene. Identification of the caveolin-3 gene as a candidate for deletion in 3p-syndrome. FEBS Lett 1999; 452: 177–180.
McNally EM, de Sa Moreira E, Duggan DJ et al: Caveolin-3 in muscular dystrophy. Hum Mol Genet 1998; 7: 871–877.
Kubisch C, Schoser BG, von During M et al: Homozygous mutations in caveolin-3 cause a severe form of rippling muscle disease. Ann Neurol 2003; 53: 512–520.
Muller JS, Piko H, Schoser BG et al: Novel splice site mutation in the caveolin-3 gene leading to autosomal recessive limb girdle muscular dystrophy. Neuromuscul Disord 2006; 16: 432–436.
Ueyama H, Horinouchi H, Obayashi K, Hashinaga M, Okazaki T, Kumamoto T : Novel homozygous mutation of the caveolin-3 gene in rippling muscle disease with extraocular muscle paresis. Neuromuscul Disord 2007; 17: 558–561.
Traverso M, Bruno C, Broccolini A et al: Truncation of Caveolin-3 causes autosomal-recessive Rippling Muscle Disease. J Neurol Neurosurg Psychiatry 2008; 79: 735–737.
Traverso M, Gazzerro E, Assereto S et al: Caveolin-3 T78M and T78K missense mutations lead to different phenotypes in vivo and in vitro. Lab Invest 2008; 88: 275–283.
Fulizio L, Nascimbeni AC, Fanin M et al: Molecular and muscle pathology in a series of caveolinopathy patients. Hum Mutat 2005; 25: 82–89.
Vatta M, Ackerman MJ, Ye B et al: Mutant caveolin-3 induces persistent late sodium current and is associated with long-QT syndrome. Circulation 2006; 114: 2104–2112.
Cronk LB, Ye B, Kaku T et al: Novel mechanism for sudden infant death syndrome: persistent late sodium current secondary to mutations in caveolin-3. Heart Rhythm 2007; 4: 161–166.
Aboumousa A, Hoogendijk J, Charlton R et al: Caveolinopathy--new mutations and additional symptoms. Neuromuscul Disord 2008; 18: 572–578.
Guglieri M, Straub V, Bushby K, Lochmuller H : Limb-girdle muscular dystrophies. Curr Opin Neurol 2008; 21: 576–584.
Guglieri M, Magri F, D'Angelo MG et al: Clinical, molecular, and protein correlations in a large sample of genetically diagnosed Italian limb girdle muscular dystrophy patients. Hum Mutat 2008; 29: 258–266.
Minetti C, Sotgia F, Bruno C et al: Mutations in the caveolin-3 gene cause autosomal dominant limb-girdle muscular dystrophy. Nat Genet 1998; 18: 365–368.
Kunkel L : Caveolin-3 deficiency as a cause of limb-girdle muscular dystrophy. J Child Neurol 1999; 14: 33–34.
Matsuda C, Hayashi YK, Ogawa M et al: The sarcolemmal proteins dysferlin and caveolin-3 interact in skeletal muscle. Hum Mol Genet 2001; 10: 1761–1766.
Figarella-Branger D, Pouget J, Bernard R et al: Limb-girdle muscular dystrophy in a 71-year-old woman with an R27Q mutation in the CAV3 gene. Neurology 2003; 61: 562–564.
Sugie K, Murayama K, Noguchi S et al: Two novel CAV3 gene mutations in Japanese families. Neuromuscul Disord 2004; 14: 810–814.
Woodman SE, Sotgia F, Galbiati F, Minetti C, Lisanti MP : Caveolinopathies: mutations in caveolin-3 cause four distinct autosomal dominant muscle diseases. Neurology 2004; 62: 538–543.
Fee DB, So YT, Barraza C, Figueroa KP, Pulst SM : Phenotypic variability associated with Arg26Gln mutation in caveolin3. Muscle Nerve 2004; 30: 375–378.
Fischer D, Schroers A, Blumcke I et al: Consequences of a novel caveolin-3 mutation in a large German family. Ann Neurol 2003; 53: 233–241.
Carbone I, Bruno C, Sotgia F et al: Mutation in the CAV3 gene causes partial caveolin-3 deficiency and hyperCKemia. Neurology 2000; 54: 1373–1376.
Reijneveld JC, Ginjaar IB, Frankhuizen WS, Notermans NC : CAV3 gene mutation analysis in patients with idiopathic hyper-CK-emia. Muscle Nerve 2006; 34: 656–658.
Merlini L, Carbone I, Capanni C et al: Familial isolated hyperCKaemia associated with a new mutation in the caveolin-3 (CAV-3) gene. J Neurol Neurosurg Psychiatry 2002; 73: 65–67.
Alias L, Gallano P, Moreno D et al: A novel mutation in the caveolin-3 gene causing familial isolated hyperCKaemia. Neuromuscul Disord 2004; 14: 321–324.
Betz RC, Schoser BG, Kasper D et al: Mutations in CAV3 cause mechanical hyperirritability of skeletal muscle in rippling muscle disease. Nat Genet 2001; 28: 218–219.
Dotti MT, Malandrini A, Gambelli S, Salvadori C, De Stefano N, Federico A : A new missense mutation in caveolin-3 gene causes rippling muscle disease. J Neurol Sci 2006; 243: 61–64.
Vorgerd M, Ricker K, Ziemssen F et al: A sporadic case of rippling muscle disease caused by a de novo caveolin-3 mutation. Neurology 2001; 57: 2273–2277.
Bae JS, Ki CS, Kim JW et al: A novel in-frame deletion in the CAV3 gene in a Korean patient with rippling muscle disease. J Neurol Sci 2007; 260: 275–278.
Kubisch C, Ketelsen UP, Goebel I, Omran H : Autosomal recessive rippling muscle disease with homozygous CAV3 mutations. Ann Neurol 2005; 57: 303–304.
Lorenzoni PJ, Scola RH, Vieira N, Vainzof M, Carsten AL, Werneck LC : A novel missense mutation in the caveolin-3 gene in rippling muscle disease. Muscle Nerve 2007; 36: 258–260.
Madrid RE, Kubisch C, Hays AP : Early-onset toe walking in rippling muscle disease due to a new caveolin-3 gene mutation. Neurology 2005; 65: 1301–1303.
Schara U, Vorgerd M, Popovic N, Schoser BG, Ricker K, Mortier W : Rippling muscle disease in childhood. J Child Neurol 2002; 17: 483–490.
Van den Bergh PY, Gerard JM, Elosegi JA, Manto MU, Kubisch C, Schoser BG : Novel missense mutation in the caveolin-3 gene in a Belgian family with rippling muscle disease. J Neurol Neurosurg Psychiatry 2004; 75: 1349–1351.
Tateyama M, Aoki M, Nishino I et al: Mutation in the caveolin-3 gene causes a peculiar form of distal myopathy. Neurology 2002; 58: 323–325.
Gonzalez-Perez P, Gallano P, Gonzalez-Quereda L et al: Phenotypic variability in a Spanish family with a Caveolin-3 mutation. J Neurol Sci 2009; 276: 95–98.
Hayashi T, Arimura T, Ueda K et al: Identification and functional analysis of a caveolin-3 mutation associated with familial hypertrophic cardiomyopathy. Biochem Biophys Res Commun 2004; 313: 178–184.
Cagliani R, Bresolin N, Prelle A et al: A CAV3 microdeletion differentially affects skeletal muscle and myocardium. Neurology 2003; 61: 1513–1519.
Yabe I, Kawashima A, Kikuchi S et al: Caveolin-3 gene mutation in Japanese with rippling muscle disease. Acta Neurol Scand 2003; 108: 47–51.
Galbiati F, Volonte D, Minetti C, Bregman DB, Lisanti MP : Limb-girdle muscular dystrophy (LGMD-1C) mutants of caveolin-3 undergo ubiquitination and proteasomal degradation. Treatment with proteasomal inhibitors blocks the dominant negative effect of LGMD-1C mutanta and rescues wild-type caveolin-3. J Biol Chem 2000; 275: 37702–37711.
Galbiati F, Volonte D, Minetti C, Chu JB, Lisanti MP : Phenotypic behavior of caveolin-3 mutations that cause autosomal dominant limb girdle muscular dystrophy (LGMD-1C). Retention of LGMD-1C caveolin-3 mutants within the golgi complex. J Biol Chem 1999; 274: 25632–25641.
Smythe GM, Rando TA : Altered caveolin-3 expression disrupts PI(3) kinase signaling leading to death of cultured muscle cells. Exp Cell Res 2006; 312: 2816–2825.
Ohsawa Y, Hagiwara H, Nakatani M et al: Muscular atrophy of caveolin-3-deficient mice is rescued by myostatin inhibition. J Clin Invest 2006; 116: 2924–2934.
Hernandez-Deviez DJ, Howes MT, Laval SH, Bushby K, Hancock JF, Parton RG : Caveolin regulates endocytosis of the muscle repair protein, dysferlin. J Biol Chem 2008; 283: 6476–6488.
Matsuda C, Aoki M, Hayashi YK, Ho MF, Arahata K, Brown Jr RH : Dysferlin is a surface membrane-associated protein that is absent in Miyoshi myopathy. Neurology 1999; 53: 1119–1122.
Mercier I, Jasmin JF, Pavlides S et al: Clinical and translational implications of the caveolin gene family: lessons from mouse models and human genetic disorders. Lab Invest 2009; 89: 614–623.
Gratton JP, Lin MI, Yu J et al: Selective inhibition of tumor microvascular permeability by cavtratin blocks tumor progression in mice. Cancer Cell 2003; 4: 31–39.
Lin MI, Yu J, Murata T, Sessa WC : Caveolin-1-deficient mice have increased tumor microvascular permeability, angiogenesis, and growth. Cancer Res 2007; 67: 2849–2856.
Jasmin JF, Mercier I, Dupuis J, Tanowitz HB, Lisanti MP : Short-term administration of a cell-permeable caveolin-1 peptide prevents the development of monocrotaline-induced pulmonary hypertension and right ventricular hypertrophy. Circulation 2006; 114: 912–920.
Acknowledgements
This work was supported by AFM Grant no. 13771 and by MIUR. MPL and FS were supported by grants from the Muscular Dystrophy Association(MDA, USA) and the American Heart Association (AHA) (to MPL).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gazzerro, E., Sotgia, F., Bruno, C. et al. Caveolinopathies: from the biology of caveolin-3 to human diseases. Eur J Hum Genet 18, 137–145 (2010). https://doi.org/10.1038/ejhg.2009.103
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ejhg.2009.103
Keywords
This article is cited by
-
Loss of dysferlin or myoferlin results in differential defects in excitation–contraction coupling in mouse skeletal muscle
Scientific Reports (2021)
-
Caveolin1 Tyrosine-14 Phosphorylation: Role in Cellular Responsiveness to Mechanical Cues
The Journal of Membrane Biology (2020)
-
Dystrophy-associated caveolin-3 mutations reveal that caveolae couple IL6/STAT3 signaling with mechanosensing in human muscle cells
Nature Communications (2019)
-
Biochemical and pathological changes result from mutated Caveolin-3 in muscle
Skeletal Muscle (2018)
-
A review of the role of cav-1 in neuropathology and neural recovery after ischemic stroke
Journal of Neuroinflammation (2018)