Abstract
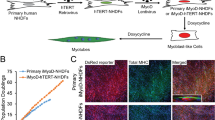
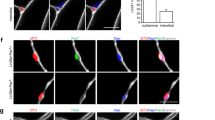
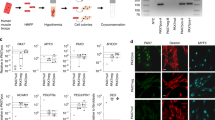
In vitro culture systems of human myogenic cells contribute greatly to elucidation of the molecular mechanisms underlying terminal myogenic differentiation and symptoms of neuromuscular diseases. However, human myogenic cells have limited ability to proliferate in culture. We have established an improved immortalization protocol for human myogenic cells derived from healthy and diseased muscles; constitutive expression of mutated cyclin-dependent kinase 4, cyclin D1 and telomerase immortalized human myogenic cells. Normal diploid chromosomes were preserved after immortalization. The immortalized human myogenic cells divided as rapidly as primary human myogenic cells during the early passages, and underwent myogenic, osteogenic and adipogenic differentiation under appropriate culture conditions. The immortalized cells contributed to muscle differentiation upon xenotransplantation to immunodeficient mice under conditions of regeneration following muscle injury. We also succeeded in immortalizing cryopreserved human myogenic cells derived from Leigh disease patients following primary culture. Forced expression of the three genes shortened their cell cycle to <30 h, which is similar to the doubling time of primary cultured human myogenic cells during early passages. The immortalization protocol described here allowed human myogenic cells to recapture high proliferation activity without compromising their differentiation potential and normal diploidy.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout









Similar content being viewed by others
References
Kuang S, Charge SB, Seale P, Huh M, Rudnicki MA . Distinct roles for Pax7 and Pax3 in adult regenerative myogenesis. J Cell Biol 2006; 172: 103–113.
Oustanina S, Hause G, Braun T . Pax7 directs postnatal renewal and propagation of myogenic satellite cells but not their specification. EMBO J 2004; 23: 3430–3439.
Yaffe D, Saxel O . Serial passaging and differentiation of myogenic cells isolated from dystrophic mouse muscle. Nature 1977; 270: 725–727.
Wada MR, Inagawa-Ogashiwa M, Shimizu S, Yasumoto S, Hashimoto N . Generation of different fates from multipotent muscle stem cells. Development 2002; 129: 2987–2995.
Mukai A, Hashimoto N . Localized cyclic AMP-dependent protein kinase activity is required for myogenic cell fusion. Exp Cell Res 2008; 314: 387–397.
Decary S, Mouly V, Hamida CB, Sautet A, Barbet JP, Butler-Browne GS . Replicative potential and telomere length in human skeletal muscle: implications for satellite cell-mediated gene therapy. Hum Gene Ther 1997; 8: 1429–1438.
Hashimoto N, Kiyono T, Wada MR, Umeda R, Goto Y, Nonaka I et al. Osteogenic properties of human myogenic progenitor cells. Mech Dev 2008; 125: 257–269.
Bigot A, Jacquemin V, Debacq-Chainiaux F, Butler-Browne GS, Toussaint O, Furling D et al. Replicative aging down-regulates the myogenic regulatory factors in human myoblasts. Biol Cell 2008; 100: 189–199.
Hashimoto N, Kiyono T, Wada MR, Shimizu S, Yasumoto S, Inagawa M . Immortalization of human myogenic progenitor cell clone retaining multipotentiality. Biochem Biophys Res Commun 2006; 348: 1383–1388.
Seigneurin-Venin S, Bernard V, Tremblay JP . Telomerase allows the immortalization of T antigen-positive DMD myoblasts: a new source of cells for gene transfer application. Gene Therapy 2000; 7: 619–623.
Kiyono T, Foster SA, Koop JI, McDougall JK, Galloway DA, Klingelhutz AJ . Both Rb/p16INK4a inactivation and telomerase activity are required to immortalize human epithelial cells. Nature 1998; 396: 84–88.
Gorbunova V, Seluanov A, Pereira-Smith OM . Expression of human telomerase (hTERT) does not prevent stress-induced senescence in normal human fibroblasts but protects the cells from stress-induced apoptosis and necrosis. J Biol Chem 2002; 277: 38540–38549.
Zhu CH, Mouly V, Cooper RN, Mamchaoui K, Bigot A, Shay JW et al. Cellular senescence in human myoblasts is overcome by human telomerase reverse transcriptase and cyclin-dependent kinase 4: consequences in aging muscle and therapeutic strategies for muscular dystrophies. Aging Cell 2007; 6: 515–523.
Cudre-Mauroux C, Occhiodoro T, Konig S, Salmon P, Bernheim L, Trono D . Lentivector-mediated transfer of Bmi-1 and telomerase in muscle satellite cells yields a Duchenne myoblast cell line with long-term genotypic and phenotypic stability. Hum Gene Ther 2003; 14: 1525–1533.
Mukai A, Kurisaki T, Sato SB, Kobayashi T, Kondoh G, Hashimoto N . Dynamic clustering and dispersion of lipid rafts contribute to fusion competence of myogenic cells. Exp Cell Res 2009; 315: 3052–3063.
Renault V, Thornell LE, Eriksson PO, Butler-Browne G, Mouly V . Regenerative potential of human skeletal muscle during aging. Aging Cell 2002; 1: 132–139.
Sajko S, Kubinova L, Cvetko E, Kreft M, Wernig A, Erzen I . Frequency of M-cadherin-stained satellite cells declines in human muscles during aging. J Histochem Cytochem 2004; 52: 179–185.
Wright WE, Shay JW . Historical claims and current interpretations of replicative aging. Nat Biotechnol 2002; 20: 682–688.
Shay JW, Wright WE . Telomeres and telomerase: implications for cancer and aging. Radiat Res 2001; 155 (1 Part 2): 188–193.
Shay JW, Wright WE . Senescence and immortalization: role of telomeres and telomerase. Carcinogenesis 2005; 26: 867–874.
Toussaint O, Medrano EE, von Zglinicki T . Cellular and molecular mechanisms of stress-induced premature senescence (SIPS) of human diploid fibroblasts and melanocytes. Exp Gerontol 2000; 35: 927–945.
Haga K, Ohno S, Yugawa T, Narisawa-Saito M, Fujita M, Sakamoto M et al. Efficient immortalization of primary human cells by p16INK4a-specific short hairpin RNA or Bmi-1, combined with introduction of hTERT. Cancer Sci 2007; 98: 147–154.
Bodnar AG, Ouellette M, Frolkis M, Holt SE, Chiu CP, Morin GB et al. Extension of life-span by introduction of telomerase into normal human cells. Science 1998; 279: 349–352.
Ramirez RD, Sheridan S, Girard L, Sato M, Kim Y, Pollack J et al. Immortalization of human bronchial epithelial cells in the absence of viral oncoproteins. Cancer Res 2004; 64: 9027–9034.
Ramirez RD, Morales CP, Herbert BS, Rohde JM, Passons C, Shay JW et al. Putative telomere-independent mechanisms of replicative aging reflect inadequate growth conditions. Genes Dev 2001; 15: 398–403.
Carlson BM, Faulkner JA . Muscle transplantation between young and old rats: age of host determines recovery. Am J Physiol 1989; 256 (6 Part 1): C1262–C1266.
Benbassat CA, Maki KC, Unterman TG . Circulating levels of insulin-like growth factor (IGF) binding protein-1 and -3 in aging men: relationships to insulin, glucose, IGF, and dehydroepiandrosterone sulfate levels and anthropometric measures. J Clin Endocrinol Metab 1997; 82: 1484–1491.
Doherty TJ, Vandervoort AA, Brown WF . Effects of ageing on the motor unit: a brief review. Can J Appl Physiol 1993; 18: 331–358.
Rando TA, Blau HM . Primary mouse myoblast purification, characterization, and transplantation for cell-mediated gene therapy. J Cell Biol 1994; 125: 1275–1287.
Decary S, Hamida CB, Mouly V, Barbet JP, Hentati F, Butler-Browne GS . Shorter telomeres in dystrophic muscle consistent with extensive regeneration in young children. Neuromuscul Disord 2000; 10: 113–120.
Rao SS, Kohtz DS . Positive and negative regulation of D-type cyclin expression in skeletal myoblasts by basic fibroblast growth factor and transforming growth factor beta. A role for cyclin D1 in control of myoblast differentiation. J Biol Chem 1995; 270: 4093–4100.
Guo K, Walsh K . Inhibition of myogenesis by multiple cyclin-Cdk complexes. Coordinate regulation of myogenesis and cell cycle activity at the level of E2F. J Biol Chem 1997; 272: 791–797.
Hashimoto N, Ogashiwa M, Iwashita S . Role of tyrosine kinase in the regulation of myogenin expression. Eur J Biochem 1995; 227: 379–387.
Hashimoto N, Ogashiwa M, Okumura E, Endo T, Iwashita S, Kishimoto T . Phosphorylation of a proline-directed kinase motif is responsible for structural changes in myogenin. FEBS Lett 1994; 352: 236–242.
Sasaki R, Narisawa-Saito M, Yugawa T, Fujita M, Tashiro H, Katabuchi H et al. Oncogenic transformation of human ovarian surface epithelial cells with defined cellular oncogenes. Carcinogenesis 2009; 30: 423–431.
Miyoshi H . Gene delivery to hematopoietic stem cells using lentiviral vectors. Methods Mol Biol 2004; 246: 429–438.
Imabayashi H, Mori T, Gojo S, Kiyono T, Sugiyama T, Irie R et al. Redifferentiation of dedifferentiated chondrocytes and chondrogenesis of human bone marrow stromal cells via chondrosphere formation with expression profiling by large-scale cDNA analysis. Exp Cell Res 2003; 288: 35–50.
Hirano H, Watanabe T . Microsequencing of proteins electrotransferred onto immobilizing matrices from polyacrylamide gel electrophoresis: application to an insoluble protein. Electrophoresis 1990; 11: 573–580.
Bader D, Masaki T, Fischman DA . Immunochemical analysis of myosin heavy chain during avian myogenesis in vivo and in vitro. J Cell Biol 1982; 95: 763–770.
Saito Y, Nonaka I, Qu Z, Balkir L, van Deutekom JC, Robbins PD et al. Initiation of satellite cell replication in bupivacaine-induced myonecrosis. Acta Neuropathol (Berl) 1994; 88: 252–257.
Furukawa Y, Hashimoto N, Yamakuni T, Ishida Y, Kato C, Ogashiwa M et al. Down-regulation of an ankyrin repeat-containing protein, V-1, during skeletal muscle differentiation and its re-expression in the regenerative process of muscular dystrophy. Neuromuscul Disord 2003; 13: 32–41.
Acknowledgements
We thank H Miyoshi for providing lentivirus vectors. This study was supported by grants to NH and TK from the Ministry of Health, Labor and Welfare of Japan.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on Gene Therapy website
Rights and permissions
About this article
Cite this article
Shiomi, K., Kiyono, T., Okamura, K. et al. CDK4 and cyclin D1 allow human myogenic cells to recapture growth property without compromising differentiation potential. Gene Ther 18, 857–866 (2011). https://doi.org/10.1038/gt.2011.44
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/gt.2011.44
Keywords
This article is cited by
-
Simple and efficient differentiation of human iPSCs into contractible skeletal muscles for muscular disease modeling
Scientific Reports (2023)
-
Puromycin-based purification of cells with high expression of the cytochrome P450 CYP3A4 gene from a patient with drug-induced liver injury (DILI)
Stem Cell Research & Therapy (2022)
-
In vitro maturation of Toxoplasma gondii bradyzoites in human myotubes and their metabolomic characterization
Nature Communications (2022)
-
Regulation of A-to-I RNA editing and stop codon recoding to control selenoprotein expression during skeletal myogenesis
Nature Communications (2022)
-
Immortalization of cells derived from domestic dogs through expressing mutant cyclin-dependent kinase 4, cyclin D1, and telomerase reverse transcriptase
Cytotechnology (2022)