Abstract
Background:
Bone morphogenetic protein-3b (BMP-3b) is a member of the transforming growth factor-β (TGF-β) superfamily. BMP-3b regulates osteogenesis and has critical roles in developing embryos. BMP-3b is expressed not only in the bone and developing embryos but also in adipose tissues. However, the functions of BMP-3b in adipose tissue are still unknown.
Methods:
BMP-3b expression was quantified in various adipose tissues and in the adipose-derived stromal-vascular fraction (SVF) and mature adipocyte fraction (AD.F) of mice. We also used 3T3-L1 preadipocytes to analyze the expression, function and molecular forms of BMP-3b. In order to determine the effects of BMP-3b on the adipogenesis of 3T3-L1 cells, BMP-3b siRNA-mediated knockdown and gene overexpression studies were performed, and a conditioned medium (CM) containing the BMP-3b protein was added to 3T3-L1 cell cultures. Adipocyte differentiation was evaluated by measuring the expression of adipogenic markers or by Oil Red O staining. The molecular form of BMP-3b that was secreted from the 3T3-L1 cells was analyzed by western blotting.
Results:
BMP-3b is expressed in all adipose tissues and is expressed at higher levels in preadipocytes than in mature adipocytes. In mesenteric adipose tissue, BMP-3b expression was increased in diet-induced obesity (DIO) mice as compared with that in control mice. BMP-3b was also expressed highly in 3T3-L1 cells. We showed that siRNA-mediated knockdown of endogenous BMP-3b expression in 3T3-L1 cells enhanced adipogenesis. Conversely, overexpressing BMP-3b inhibited adipocyte differentiation. We also showed that addition of CM containing the BMP-3b protein inhibited the differentiation of 3T3-L1 cells, and that this inhibitory effect was abolished by removing BMP-3b with an anti-BMP-3b antibody. Furthermore, BMP-3b was secreted from adipocytes as a unique non-covalent complex.
Conclusion:
These data suggest that BMP-3b is secreted from adipocytes and is involved in adipocyte differentiation.
Similar content being viewed by others
Introduction
Adipose tissue has a critical role in energy balance, and therefore contributes to the development of obesity. Recent studies have shown that adipose tissue functions as an endocrine organ and secretes various factors, such as adiponectin, leptin, resistin and tumor necrosis factor-α.1, 2, 3 These factors have important autocrine/paracrine roles in regulating adipocyte differentiation and metabolism, and their expression or secretion level is affected by the degree of adiposity. The identification and characterization of novel factors will enhance our understanding of the function of adipose tissue, including adipogenesis.
The members of the bone morphogenetic protein (BMP) family are a subgroup of the transforming growth factor-β (TGF-β) superfamily.4, 5 We originally isolated BMP-3b (also called growth/differentiation factor-10) from a rat femur, and subsequent studies have shown that BMP-3b inhibits osteoblast differentiation.6, 7, 8, 9 In osteoblasts, BMP-3b transcription correlates with differentiation. It has been reported that BMP-3b is augmented by hypoxia in chondrocytes, a process that is regulated by SOX9;10 is repressed by DNA promoter methylation and has tumor-suppressive functions in lung cancer and malignant mesothelioma.11, 12 Previously, we showed that BMP-3b is essential for head formation in Xenopus embryos and acts as a dorsalizing factor.13 BMP-3b also interacts with other BMP family members, such as BMP-2 and nodal-like protein.13
BMPs are synthesized as precursor proteins that are proteolytically cleaved to generate an N-terminal pro-region and a C-terminal mature region.4, 5, 9 In general, BMPs are biologically active as dimers of the mature region.4, 5, 9 The mature region of BMP-3b and BMP-3, which are in the same BMP subgroup, share approximately 80% amino-acid sequence identity and both proteins have the same functions in osteoblasts and embryonic development.9, 13, 14, 15 On the other hand, the pro-regions of BMP-3b and BMP-3 share only 30–35% similarity, and BMP-3b has a unique region that is required for head formation in Xenopus embryos. Thus, BMP-3b and BMP-3 possess different activities.9, 13
BMP-3b is strongly expressed not only in the bone and developing embryos but also in mammalian adipose tissue.9, 16 The expression of other BMPs in adipose tissue has not been determined, but the BMP receptors (activin receptor-like kinase-2 (ALK2), ALK3, ALK4, ALK6; BMP type-II receptor (BMPR-II); activin type-II receptor (ActR-II); and ActR-IIB) are expressed in adipocytes.17, 18 Although the secretion process and the molecular forms of BMPs secreted by adipocytes are unclear, certain BMPs, such as BMP-2 and BMP-7, have been reported to promote adipogenesis.18, 19, 20, 21, 22 However, the functions of BMP-3b and BMP-3 in adipocytes are unknown.
To elucidate the role of BMP-3b in adipose tissue, we examined the expression levels and activity of BMP-3b during adipocyte differentiation and identified the molecular forms of BMP-3b that are secreted by adipocytes. We found that BMP-3b is expressed at higher levels in pre-adipocytes than in mature adipocytes, and that its expression level is augmented in mesenteric adipose tissue from diet-induced obesity (DIO) mice. Small interfering RNA (siRNA)-mediated knockdown of BMP-3b expression enhances adipogenesis in the preadipocyte cell line 3T3-L1. By contrast, BMP-3b gene overexpression and addition of the BMP-3b protein suppress adipogenesis in 3T3-L1 cells. We also show that 3T3-L1 cells endogenously secrete biologically active BMP-3b as a unique complex that contains both the pro-region and the mature region.
Materials and methods
Quantitative RT-PCR analysis
Total RNA was extracted from tissues by using the TRIzol reagent (Invitrogen, Life Technologies Corp., Carlsbad, CA, USA) or from cells by using the RNeasy kit (Qiagen, Hamburg, Germany) according to the manufacturer's instructions. cDNA was synthesized by using a quantitative cDNA kit (Qiagen), and a portion of the cDNA was amplified by using the LightCycler System (Roche, Basel, Switzerland) and SYBR Premix Ex Taq (TaKaRa, Shiga, Japan). Gene copy numbers were derived from a standard curve by using serially diluted plasmid cDNAs and were normalized against the ribosomal protein S18 (S18) mRNA or 18S ribosomal RNA (18S). The primers are listed in Supplementary Table 1.
Animals
All experiments were conducted in accordance with the National Cerebral and Cardiovascular Center Research Institute Guide for the Care and Use of Experimental Animals. DIO C57BL/6J (B6J-DIO) and C57BL/6J mice were obtained from Jackson Laboratory (Bar Harbor, ME, USA). The B6J-DIO mice were generated by administering a high-fat diet (60 kcal% fat; D12492; Research Diet, New Brunswick, NJ, USA).
Fractionation and isolation of SVF from adipose tissues
Adipose tissue was fractionated as described previously,23 with some modifications. Briefly, fat pads were isolated from 6-week-old male C57BL/6J mice and then minced. Up to 1 g of tissue was digested with 1 mg of collagenase type-VIII (Sigma, St Louis, MO, USA) in Krebs–Ringer bicarbonate HEPES buffer containing 1% bovine serum albumin (Sigma) at 37 °C for 1 h. After centrifugation, adipocytes were obtained from the upper layer and the stromal-vascular fraction (SVF) was obtained from the precipitated cells.
Cell culture
Cells from the SVF were cultured in Dulbecco's Modified Eagle's Medium (DMEM) (Invitrogen) with 10% calf serum (Invitrogen). Two days after reaching confluence (experimental day 0), the cells were differentiated with 1 μg ml−1 of insulin, 0.5 mM 3-isobutyl-1-methylxanthine, 0.25 μM dexamethasone and 10% fetal bovine serum (FBS) (Invitrogen). On day 2, the induction medium was replaced with DMEM containing 1 μg ml−1 insulin and 10% FBS. On day 4, the medium was changed to DMEM containing 10% FBS and replaced every other day. 3T3-L1 cells were obtained from the American Type Culture Collection and subcultured in DMEM containing 10% calf serum. 3T3-L1 cells were differentiated with the SVF as described above. Chinese hamster ovary (CHO) cells were obtained from the American Type Culture Collection and maintained in α-minimum essential medium (Invitrogen) containing 10% FBS or Opti-MEM (Invitrogen) without FBS.
Small interfering RNA
An siRNA specific for mouse BMP-3b (Silencer Select Pre-designed s66568) was purchased from Ambion (Austin, TX, USA). The targeted nucleotide sequence of the siRNA was CCACATGCCCTATATCCTT. Silencer Select Negative Control #1 (Ambion) was used as a control.
Construction of expression plasmids
Plasmids encoding rat BMP-3b under the control of the SR-α promoter and Flag-tagged rat BMP-3b under the control of the cytomegalovirus (CMV) promoter were used in this study.9 The Flag tag was inserted at the N-terminus of the mature region, and the Flag-tagged BMP-3b protein had the same biological activity as untagged BMP-3b, as we reported previously.13 The SR-α-BMP-3b plasmid was used to generate CHO cells stably expressing rat BMP-3b as described below.6, 9
Transfections
3T3-L1 cells were transfected with plasmids using a Nucleofector (Lonza, Basel, Switzerland) according to the manufacturer's recommendations. Briefly, the cells were harvested and resuspended in the Nucleofector solution (100 μl) at 2–4 × 106 cells. After adding 2–10 μg of the expression plasmid, the cells were transfected by using program ‘T-30’ for preadipocytes or ‘A-33’ for adipocytes. The cells were then plated at 1 × 105 cells cm−2 and used in differentiation experiments 2 days later. CHO cells were transfected as described previously.13
For siRNA-mediated knockdown of 3T3-L1 cells, the cells were transfected with each siRNA (50 nM) using a Nucleofactor, plated at 1.25 × 105 cells cm−2 and then differentiated after 4 h.
Oil Red O staining
The cells were washed twice with phosphate-buffered saline, fixed in 10% formalin neutral buffer solution for 1 h and then stained with Oil Red O solution for 2 h at room temperature. Excess stain was removed by washing with water.
Biochemical analyses
Conditioned media from cells transfected with the BMP-3b expression plasmid were collected 3 days after transfection and immunoprecipitated by using the Flag-Tagged Protein Immunoprecipitation kit (Sigma) or an anti-BMP-3b pro-region antibody (3bpro) IgG immunoaffinity gel, as described previously.13 The conditioned medium (CM) was purified with Heparin–Sepharose (GE Healthcare, Little Chalfont, UK) (Figure 5c). An antibody specific to the pro-region of rat BMP-3b, 3bpro, was produced by immunizing rabbits with the synthetic peptide SEPPRWPRAREVFC, corresponding to amino acids 133–146 of BMP-3b. A 3bpro immunoaffinity gel was prepared by coupling AFFI-GEL 10 (Bio-Rad, Hercules, CA, USA) to the IgG fraction of 3bpro serum, as reported previously.24 The antibody against the mature region of human BMP-3b was purchased from R&D Systems (AF1543; Minneapolis, MN, USA) (Figures 4a and d, and 6b and c).
CHO cells stably expressing BMP-3b generated by the dihydrofolate reductase-coupled method were used to prepare the recombinant BMP-3b protein as described previously.6 Parental wild-type CHO cells were used as control. The CHO CM was collected and filtered through a 0.45-μm Milliex Filter Unit (Millipore, Billerica, MA, USA). A concentrated CM was prepared using Centriprep (Millipore), as described previously.13 The CM was subjected to a 3bpro antibody IgG immunoaffinity gel column, and the pass-through fraction was used for experiments (Figures 4d–f). Western blotting was performed as described previously.13 Briefly, the samples were separated on an sodium dodecyl sulfate-PAGE gel (TEFCO, Tokyo, Japan) and transferred onto nitrocellulose membranes (Bio-Rad). The membranes were probed with the appropriate antibodies and developed by using SuperSignal West Pico or Femto (Pierce, Waltham, MA, USA). Signal was detected using LAS-1000 (Fujifilm, Tokyo, Japan).
Statistical analysis
Data are expressed as the means±s.e.m. and were analyzed statistically by Student's t-test.
Results
BMP-3b is predominantly expressed in adipose tissue
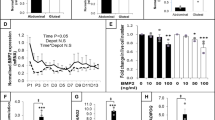
Mouse BMP-3b mRNA was highly expressed in epididymal adipose tissue, the brain, bone and aorta (Figure 1a). We used quantitative reverse transcription-PCR (RT-PCR) to examine BMP-3b expression in other adipose tissues and compared BMP-3b expression to BMP-3 and BMP-2 expression in these tissues. BMP-3b was expressed in all adipose tissues, with the highest expression in epididymal adipose tissue, where expression was approximately five times higher than that in brown adipose tissue (Figure 1b). Mouse BMP-3 and BMP-2 were expressed in all of the examined adipose tissues, but at much lower levels than that in BMP-3b (Figure 1b).
Expression of BMP-3b, BMP-3 and BMP-2 in mice. Expression levels were quantified by quantitative RT-PCR. (a) BMP-3b expression in mouse tissues. sk, skeletal. (b) Epididymal, mesenteric, perirenal, subcutaneous and brown adipose tissues from a male mouse. BAT, brown adipose tissue. (c) SVFs and Ad. Fs from mouse epididymal fat pads. **P<0.01 versus SVF. (d) Primary culture of SVF differentiation. Day 0, preadipocytes; day 8, mature adipocytes, **P<0.01 versus day 0. (a–d) a.u., copy numbers corrected according to the S18 amplification values. (e) DIO mice were generated by administering a high-fat diet for 4 weeks. The expression levels were normalized to S18 levels. Relative gene expression is shown as the ratio compared with the control expression level. DIO, diet-induced obesity; **P<0.01 versus control. (a–e) Data are represented as the means±s.e.m. of three independent experiments (n=3).
Because adipose tissue contains a mature adipocyte fraction (Ad. F) together with the SVF that contains pre-adipocytes, we separated the Ad. F from the SVF. BMP-3b was expressed at significantly higher levels in the SVF than in the Ad. F (Figure 1c). By contrast, BMP-3 expression was much lower than BMP-3b expression and was higher in the Ad. F than in the SVF (Figure 1c and Supplementary Figure 1a). BMP-2 mRNA was expressed at lower levels than BMP-3b mRNA and was comparably expressed in the SVF and the Ad. F (Figure 1c and Supplementary Figure 1a). We also examined BMP-3b expression in mature adipocytes that were differentiated (day 8) from SVF primary cultures. BMP-3b expression was detected at day 0 but decreased by day 8 (Figure 1d). By contrast, BMP-3 was undetectable at day 0 and was expressed at low levels on day 8 (Figure 1d and Supplementary Figure 1b). BMP-2 expression was lower than BMP-3b expression and was roughly equal at day 0 and day 8 (Figure 1d and Supplementary Figure 1b). Moreover, we examined the changes in BMP-3b expression in the adipose tissues of obese mice induced by high-fat diet. The BMP-3b mRNA levels were higher in the mesenteric adipose tissue of DIO mice as compared with that in control mice, whereas in other adipose tissues such as subcutaneous adipose tissue, BMP-3b expression was not obviously altered in the DIO mice (Figure 1e). In addition, the BMP-3b mRNA levels were significantly increased in the SVF from the mesenteric adipose tissue of DIO mice (Supplementary Figure 2). These results suggest that BMP-3b is involved in adipocyte differentiation.
Because our results suggested that BMP-3b is expressed in preadipocytes, we next examined BMP-3b expression during preadipocyte differentiation using a preadipocyte cell line (3T3-L1). BMP-3b was expressed in both preadipocytes (day 0) and mature adipocytes (day 8), and was expressed at higher levels in preadipocytes than in mature adipocytes (Figures 2a and b, and Supplementary Figure 3), consistent with the SVF culture results (Figures 1c and d). By contrast, BMP-2, BMP-3 and BMP-7 were not expressed in 3T3-L1 preadipocytes (day 0) or mature adipocytes (day 8), and BMP-4 and BMP-6 were weakly expressed only in preadipocytes (day 0) (Figure 2a). The expression of adipogenic markers, adipocyte fatty acid-binding protein (aP2), peroxisome proliferator-activated receptor-γ (PPARγ) and adiponectin, was induced upon 3T3-L1 cell differentiation (Figure 2c), as was reported previously.25
Expression of BMP-3b and other BMPs in 3T3-L1 cells. (a, c) RT-PCR analysis of BMP-3b, other BMPs and adipogenic markers during the differentiation of 3T3-L1 cells. The products were separated on 2% agarose gels and visualized with ethidium bromide. The positive control (P) is RNA from calvariae for BMP-3b, BMP-3 and BMP-2; lung for BMP-4 and BMP-6; and brain for BMP-7. N, negative control (H2O). (b) Quantitative RT-PCR analysis of BMP-3b in 3T3-L1 cells. The data are presented as the means±s.e.m. of three independent experiments (n=3). a.u., expression levels normalized to 18S levels. *P<0.05 versus preadipocytes. pre, preadipocytes (day 0); adipo, mature adipocytes (day 8).
BMP-3b inhibits the adipocyte differentiation of 3T3-L1 cells
To examine whether BMP-3b is involved in adipocyte differentiation, we first investigated the effects of siRNA-mediated knockdown of BMP-3b on the adipogenesis of 3T3-L1 cells because BMP-3b is highly expressed in adipocytes. The levels of endogenous BMP-3b mRNA were significantly decreased to 10% in BMP-3b siRNA-treated 3T3-L1 cells as compared with that in control siRNA-treated cells at day 0, and these inhibitory effects decreased by day 8 (Figure 3a). The BMP-3b siRNA enhanced the expression of several adipogenic marker genes (adiponectin, aP2 and PPARγ) as compared with the control at day 8 (Figure 3a), suggesting that endogenous BMP-3b inhibits adipocyte differentiation. Conversely, we overexpressed the BMP-3b gene in 3T3-L1 cells by transfection and examined the induction of adipocyte marker genes. In transfected 3T3-L1 cells, BMP-3b was overexpressed at day 0 but subsequently declined by day 8 (Figure 3b). BMP-3b overexpression suppressed the expression of adipogenic markers at day 8 (Figure 3b).
Effects of BMP-3b knockdown and overexpression on adipogenesis. (a) Effects of siRNA-mediated BMP-3b knockdown on BMP-3b expression on day 0 and day 8, and quantitative RT-PCR analysis of adipogenic markers on day 8. Relative gene expression is shown as the ratio compared with the control. si, siRNA. (b) Effects of overexpressing the BMP-3b gene. Quantitative RT-PCR analysis of BMP-3b on day 0 and day 8, and analysis of adipogenic markers on day 8. The expression levels were normalized to S18 levels. Relative gene expression is shown as the ratio compared with the control expression level. The data are expressed using a logarithm scale for BMP-3b. Mock, plasmid vector control. The data are presented as the means±s.e.m. (n=3). **P<0.01 versus control or mock. Experiments were performed three times with independent cultures.
Next, to examine the effects of the secreted BMP-3b protein on adipocyte differentiation, we used CM from stably transfected BMP-3b CHO cells (CHO/BMP-3b).6 CHO/BMP-3b was used to prepare recombinant BMP-3b protein, as reported previously.6 We confirmed that the same-size BMP-3b protein was processed and secreted from both CHO and 3T3-L1 cells, as shown in Figures 5 and 6. We used three different CM preparations in this study. CM1 contained 10% FBS, whereas CM2 contained no FBS. Because CM1 contained higher levels of secreted BMP-3b than CM2, CM2 was concentrated 10-fold to obtain CM3, which had increased levels of BMP-3b (Figure 4a). Each CM was added to the culture medium of 3T3-L1 cells (10%, vol./vol.) 2 days before induction and then every other day during adipogenesis. CHO/BMP-3b CM suppressed adiponectin expression at day 8 (Figure 4b), which was consistent with the BMP-3b knockdown and overexpression studies in 3T3-L1 cells (Figure 3). This inhibitory effect depended on the amount of BMP-3b protein in the CM. During differentiation, the expression of adipocyte markers (adiponectin, aP2, PPARγ and CCAAT-enhancer-binding protein-α (C/EBPα)) was suppressed by CM1 treatment as early as day 2 and was maintained up to day 8 (Figure 4c). We also examined the effects of CHO/BMP-3b CM on adipogenesis in a primary culture of SVF cells (Supplementary Figure 4). Similarly to 3T3-L1 cells, CM1 inhibited the expression of adiponectin, PPARγ and C/EBPα at day 8, indicating that BMP-3b suppressed the adipogenesis of SVF cells in primary culture. However, aP2 expression was not changed, which may be because of the fact that aP2 is reported to be less sensitive than PPARγ and C/EBPα as an adipogenic marker.26, 27
Effects of CM from cells stably overexpressing BMP-3b on adipogenesis. (a) CM from BMP-3b-overexpressing or wild-type CHO cells (control) was subjected to western blot analysis with an anti-BMP-3b antibody. The arrow indicates the BMP-3b protein. CM aliquots (10 μl) were subjected to western blot analysis. CM1, medium with 10% FBS; CM2, medium without FBS; CM3, 10-fold concentrated CM2. (b) Quantitative RT-PCR analysis of adiponectin on day 8 after 3T3-L1 cells were treated with each CM described in panel a. The expression levels were normalized to S18 levels. Relative gene expression is shown as the ratio compared with the control. (c) Quantitative RT-PCR analysis of adipogenic markers during adipocyte differentiation of 3T3-L1 cells that were treated with CM1. The expression levels were normalized to S18 levels. Relative gene expression is shown as the ratio compared with the expression levels of the control at day 0. (d) CM1 from panel a was subjected to a 3bpro antibody IgG immunoaffinity gel column, and the pass-through fraction was analyzed by western blotting with an anti-BMP-3b antibody. The arrow indicates the BMP-3b protein. CM aliquots (10 μl) were subjected to western blot analysis. −, CM1 before 3bpro antibody IgG immunoaffinity gel column treatment; +, the CM1 pass-through fraction of the column. (e) Oil Red O staining of differentiated 3T3-L1 cells that were incubated with CM from panel d for 8 days. (f) Quantitative RT-PCR analysis of adipogenic markers using the cells described in panel e. The expression levels were normalized to S18 levels. The relative gene expression is shown as the ratio compared with the control. (b, c, f) Data are represented as the means±s.e.m. (n=3). *P<0.05 versus control. **P<0.01 versus control; #P<0.01 versus BMP-3b CM1 or CM3. Experiments were performed three times with independent cultures.
To confirm that the effects of CHO/BMP-3b CM on 3T3-L1 cells were because of the BMP-3b protein, we examined the effects of CM that had been pre-treated using an anti-BMP-3b antibody (3bpro) IgG immunoaffinity gel. This antibody recognizes the BMP-3b protein, as shown in Figures 5 and 6. The quantity of the BMP-3b protein in the antibody-treated CM1 preparation decreased (Figure 4d), and Oil Red O staining indicated that the inhibitory effects of CM1 were virtually abolished after this treatment (Figure 4e). We also performed quantitative RT-PCR to examine the expression of various adipogenic markers (Figure 4f). aP2 expression was completely restored, but adiponectin and PPARγ expression was not completely restored, which is likely because of low levels of the BMP-3b protein in CM1 after immunodepletion (Figure 4d). These data indicate that the inhibitory effects of CM1 on the differentiation of 3T3-L1 cells are because of the BMP-3b protein.
The molecular form of BMP-3b that is secreted from 3T3-L1 cells. (a) Conditioned media from 3T3-L1 and CHO cells that were transiently transfected with a Flag-tagged BMP-3b expression plasmid were immunoprecipitated with an anti-Flag antibody and then subjected to western blot analysis with an anti-Flag antibody. IP, immunoprecipitation antibody; IB, immunoblotting antibody; mature, antibody specific to the mature region. The deduced structures and molecular weights (kDa) are shown. The precursor form consists of the pro- (open box) and the mature (closed box) regions. Mock, vector control; pre, preadipocytes; adipo, mature adipocytes. The asterisk indicates a non-specific band. Note that the anti-Flag antibody recognizes the mature region. (b) Diagram representing the BMP-3b precursor. The site (•••) used for antibody (3bpro) production is shown. The potential proteolytic processing sites are indicated by triangles. (c) CM from CHO and 3T3-L1 cells that were transfected with a BMP-3b plasmid was purified with Heparin–Sepharose and then subjected to western blot analysis with the 3bpro antibody. pro, antibody for the pro-region. (a, c) Samples were electrophoresed under reducing conditions and the results are representative of at least three independent experiments. The positions of the molecular mass markers are shown on the left (a) or in the middle (c) of the panels.
BMP-3b is secreted from 3T3-L1 cells as a non-covalent complex. (a) Conditioned media from CHO and 3T3-L1 cells that were transiently transfected with a Flag-tagged BMP-3b expression plasmid were immunoprecipitated with an anti-Flag antibody and then subjected to western blot analysis with the 3bpro antibody. IP, immunoprecipitation antibody; IB, immunoblotting antibody; mature, antibody specific for the mature region; pro, antibody specific for the pro-region. The deduced structures and molecular weights (kDa) are shown. The precursor form consists of the pro- (open box) and the mature (closed box) regions. Mock, vector control; pre, preadipocytes; adipo, mature adipocytes. The asterisk indicates a non-specific band. The samples were electrophoresed under reducing conditions. Note that the anti-Flag antibody recognizes the mature region. (b) CM from 3T3-L1 cells that were transfected with a BMP-3b expression plasmid was immunoprecipitated with a 3bpro antibody and then subjected to western blot analysis with a 3bpro antibody or an anti-BMP-3b mature antibody. (c) CM from 3T3-L1 cells was immunoprecipitated with a 3bpro antibody and then subjected to western blot analysis with an anti-BMP-3b mature antibody. (d) The biosynthetic pathway of BMP-3b in 3T3-L1 cells. The representative molecular forms and potential proteolytic processing sites (triangle) are illustrated schematically. The data are representative of at least three independent experiments.
3T3-L1 cells express a unique molecular form of BMP-3b
We showed that both exogenous addition of CHO/BMP-3b CM and BMP-3b overexpression in 3T3-L1 cells inhibited adipogenesis, suggesting that the biologically active BMP-3b protein was secreted from 3T3-L1 cells overexpressing BMP-3b. In order to understand the function of BMPs, it is necessary to analyze the secreted and molecular forms of these proteins.28, 29, 30, 31, 32 Thus, we analyzed the molecular form of BMP-3b that is secreted from 3T3-L1 cells overexpressing BMP-3b.
The cleaved mature region of BMP-3b (13 and 16 kDa) was detected in CM from both preadipocytes and adipocytes, indicating that the BMP-3b protein is processed and secreted by 3T3-L1 cells, which was similar to the findings with CHO cells (Figure 5a).
To perform a detailed characterization of the molecular form of BMP-3b, we generated an antibody specific for the pro-region of rat BMP-3b (3bpro) (Figure 5b). This antibody recognized the 60-kDa BMP-3b precursor and a broad 40-kDa pro-region in CHO CM that was purified using Heparin–Sepharose (Figure 5c, lane-2). The broad 40-kDa pro-region includes at least two pro-regions because two fragments, including the mature region (13 and 30 kDa), were cleaved from the BMP-3b precursor protein in the CHO CM (Figure 5a, lane-6). Similarly, in 3T3-L1 cells, two pro-region bands of approximately 40 kDa were detected (Figure 5c, lane-4). A predicted a 15-kDa pro-region band was also detected (Figure 5c, lanes 2 and 4), but this band was detected at lower levels than the 40-kDa pro-region band, suggesting that this band is likely not a specifically processed component. Next, we used 3bpro in immunoblot analyses of the BMP-3b precursor in CHO CM that was immunoprecipitated with an antibody specific for the mature region (anti-Flag). The 60-kDa BMP-3b precursor protein was immunoprecipitated with this antibody, but unexpectedly, the 40-kDa pro-region co-immunoprecipitated with the precursor and mature region proteins (Figure 6a, lane-2). This indicates that the BMP-3b pro-region forms a non-covalent complex with the mature and/or the precursor protein. Because this molecular complex is atypical for BMPs, we investigated whether BMP-3b was secreted from 3T3-L1 cells as a complex of the pro- and mature regions. 3T3-L1 (preadipocytes and adipocytes) CM was immunoprecipitated with an antibody specific for the mature region (anti-Flag) and then immunoblotted with 3bpro. Similar to the results obtained with CHO cells, the 40-kDa BMP-3b pro-region was detected (Figure 6a, lanes 2, 4 and 6), indicating that BMP-3b is secreted from 3T3-L1 cells (preadipocytes and adipocytes) as a complex of the mature region and the pro-region. The components of this BMP-3b complex were confirmed by immunoprecipitation with 3bpro (Figure 6b, lanes 2 and 4), and the bands that were detected with this antibody were the same size as the bands detected using the anti-Flag antibody (Figure 5a, lane-2 and Figure 6a, lane-4).
To determine whether the pro-region of BMP-3b can dimerize, we analyzed the same samples under non-reducing conditions (Supplementary Figure 5). A 40-kDa pro-region band and three bands of approximately 25 kDa corresponding to the mature region were detected, indicating that the pro-region and the mature region are secreted from 3T3-L1 cells as a monomer and dimers, respectively. Moreover, this BMP-3b non-covalent complex was verified by a chemical cross-linking method (data not shown).
To examine the endogenous BMP-3b complex, we analyzed the CM of untransfected 3T3-L1 cells. We used 20-fold larger amounts of CM for this analysis because we did not detect the complex when using the quantity of CM that is typically used in this study (for example, Figure 6b). As shown in Figure 6c, the mature region (13 and 16 kDa) immunoprecipitated with the pro-region antibody, indicating that endogenous BMP-3b was secreted from 3T3-L1 cells as a complex.
These data indicate that the secreted molecular form of BMP-3b is a unique non-covalent complex of the pro-region monomer and the mature region dimer (Figure 6d).
Discussion
To date, the functions of BMP-3b have only been studied in osteoblasts, developing embryos and lung cancer, despite the fact that BMP-3b is expressed in variety of organs, including adipose tissue. In this report, we showed that BMP-3b is expressed in adipocytes and inhibits adipogenesis as a unique complex.
We found that BMP-3b is expressed at higher levels in pre-adipocytes than in mature adipocytes, both in mouse adipose tissue, and in 3T3-L1 cells (Figures 1 and 2). We also showed that the BMP-3b transcript was the most abundant BMP, suggesting that BMP-3b is a major factor among the BMP family members in these cells. Additionally, the BMP-3b expression level was increased in mesenteric adipose tissue from DIO mice, suggesting that BMP-3b may be involved in adipogenesis.
Our functional analyses showed that siRNA-mediated knockdown of endogenous BMP-3b expression in 3T3-L1 cells enhances adipogenesis and that addition of exogenous BMP-3b protein and forced BMP-3b expression inhibit adipocyte differentiation (Figures 3 and 4). These results suggest that endogenous BMP-3b inhibits adipogenesis in these cells in an autocrine manner. Given that BMP-3b expression was decreased in mature adipocytes as compared with that in preadipocytes, these results suggest that this decrease in BMP-3b expression may be required for adipogenesis. We also showed that BMP-3b treatment suppressed the expression of PPARγ and C/EBPα, which are master transcriptional regulators of the entire terminal differentiation process,2 indicating that BMP-3b inhibited adipocyte differentiation by affecting this transcriptional cascade. In addition, we determined that DIO increased BMP-3b expression in the mesenteric adipose tissue of mice, suggesting that BMP-3b might function by a feedback mechanism to inhibit adipogenesis in abdominal obesity.
The inhibitory effects of BMP-3b on adipogenesis contrast with the stimulatory activity of other BMPs, such as BMP-2 and BMP-7, suggesting that BMP-3b may interfere with other BMPs during adipocyte differentiation by mechanisms that are similar to those that were reported previously to occur during embryogenesis and osteogenesis.9, 13 We also showed that the mature regions of BMP-3b and BMP-3 have similar activities in embryogenesis and osteogenesis, as expected from their sequence similarity in that region.9, 13 Although some studies have examined the mechanisms of action of BMP-3,9, 14, 15, 33, 34 there are inconsistencies in these data and the BMP-3 signaling pathway is still unclear. These studies show that BMP-3 binds to ActR-IIB14, 15, 33 and activates TGF-β-like signaling through this receptor (ALK4/ActR-IIB) in mammalian cells.14 Moreover, we showed that BMP receptors (for example, ALK4 and ActR-IIB) are expressed in 3T3-L1 cells and in the SVF (Supplementary Figure 6), as reported previously.17, 18 In our preliminary studies, we found that CHO/BMP-3b CM activates Smad2, which is involved in the TGF-β/activin pathway, in 3T3-L1 cells (data not shown). Therefore, BMP-3b, like BMP-3, might bind to ALK4/ActR-IIB and activate the TGF-β/activin pathway. However, the precise mechanisms by which BMP-3b regulates adipogenesis remain to be elucidated, and future studies should therefore examine the BMP-3b signal transduction pathway during adipogenesis.
In this study, we showed that BMP-3b is produced in adipocytes and that its precursor is processed to generate the pro-region and mature region. The pro-region and mature region are assembled to form a biologically active BMP-3b non-covalent complex, which is secreted from cells and is involved in adipocyte differentiation (Figure 6d). The secretion process and the secreted molecular forms of other BMPs from adipocytes have not been analyzed, although BMP-2 and BMP-7 are known to function in adipogenesis.18, 19, 20, 21, 22 Therefore, to our knowledge, BMP-3b is the first BMP family member that has been shown to be secreted from adipocytes as a non-covalent complex. Moreover, we determined that the BMP-3b non-covalent complex is also present in the fat tissues of BMP-3b transgenic mice (data not shown), suggesting that this complex is a basic structure required for the function of BMP-3b in vivo. Future studies will need to examine recombinant BMP-3b complexes in order to clarify the precise molecular mechanisms that underlie their function.
In conclusion, our results show that BMP-3b is expressed in adipocytes, regulates adipocyte differentiation in an autocrine manner and is secreted by adipocytes as a unique non-covalent complex. Our data provide new insight into the mechanisms of adipogenesis as well as the causes of obesity. Because this study was performed predominantly in vitro, we are currently investigating the physiological and pathophysiological significance of BMP-3b in adipose tissues in vivo using an animal model of obesity and BMP-3b-null or transgenic mice.
References
Rosen ED, MacDougald OA . Adipocyte differentiation from the inside out. Nat Rev Mol Cell Biol 2006; 7: 885–896.
Farmer SR . Transcriptional control of adipocyte formation. Cell Metab 2006; 4: 263–273.
Kadowaki T, Yamauchi T . Adiponectin and adiponectin receptors. Endocr Rev 2005; 26: 439–451.
Chen D, Zhao M, Mundy GR . Bone morphogenetic proteins. Growth Factors 2004; 22: 233–241.
Feng XH, Derynck R . Specificity and versatility in TGF-beta signaling through Smads. Annu Rev Cell Dev Biol 2005; 21: 659–693.
Takao M, Hino J, Takeshita N, Konno Y, Nishizawa T, Matsuo H et al. Identification of rat bone morphogenetic protein-3b (BMP-3b), a new member of BMP-3. Biochem Biophys Res Commun 1996; 219: 656–662.
Hino J, Takao M, Takeshita N, Konno Y, Nishizawa T, Matsuo H et al. cDNA cloning and genomic structure of human bone morphogenetic protein-3b (BMP-3b). Biochem Biophys Res Commun 1996; 223: 304–310.
Hino J, Matsuo H, Kangawa K . Bone morphogenetic protein-3b (BMP-3b) gene expression is correlated with differentiation in rat calvarial osteoblasts. Biochem Biophys Res Commun 1999; 256: 419–424.
Hino J, Kangawa K, Matsuo H, Nohno T, Nishimatsu S . Bone morphogenetic protein-3 family members and their biological functions. Front Biosci 2004; 9: 1520–1529.
Lafont JE, Talma S, Hopfgarten C, Murphy CL . Hypoxia promotes the differentiated human articular chondrocyte phenotype through SOX9-dependent and -independent pathways. J Biol Chem 2008; 283: 4778–4786.
Dai ZY, Popkie AP, Zhu WG, Timmers CD, Raval A, Tannehill-Gregg S et al. Bone morphogenetic protein 3B silencing in non-small-cell lung cancer. Oncogene 2004; 23: 3521–3529.
Kimura K, Toyooka S, Tsukuda K, Yamamoto H, Suehisa H, Soh J et al. The aberrant promoter methylation of BMP3b and BMP6 in malignant pleural mesotheliomas. Oncol Rep 2008; 20: 1265–1268.
Hino J, Nishimatsu S, Nagai T, Matsuo H, Kangawa K, Nohno T . Coordination of BMP-3b and cerberus is required for head formation of Xenopus embryos. Dev Biol 2003; 260: 138–157.
Daluiski A, Engstrand T, Bahamonde ME, Gamer LW, Agius E, Stevenson SL et al. Bone morphogenetic protein-3 is a negative regulator of bone density. Nat Genet 2001; 27: 84–88.
Gamer LW, Nove J, Levin M, Rosen V . BMP-3 is a novel inhibitor of both activin and BMP-4 signaling in Xenopus embryos. Dev Biol 2005; 285: 156–168.
Zhao RB, Lawler AM, Lee SJ . Characterization of GDF-10 expression patterns and null mice. Dev Biol 1999; 212: 68–79.
Hirai S, Yamanaka M, Kawachi H, Matsui T, Yano H . Activin A inhibits differentiation of 3T3-L1 preadipocyte. Mol Cell Endocrinol 2005; 232: 21–26.
Tseng YH, Kokkotou E, Schulz TJ, Huang TL, Winnay JN, Taniguchi CM et al. New role of bone morphogenetic protein 7 in brown adipogenesis and energy expenditure. Nature 2008; 454: 1000–1004.
Wang EA, Israel DI, Kelly S, Luxenberg DP . Bone morphogenetic protein-2 causes commitment and differentiation in C3H10T1/2 and 3T3 cells. Growth Factors 1993; 9: 57–71.
Chen D, Ji X, Harris MA, Feng JQ, Karsenty G, Celeste AJ et al. Differential roles for bone morphogenetic protein (BMP) receptor type IB and IA in differentiation and specification of mesenchymal precursor cells to osteoblast and adipocyte lineages. J Cell Biol 1998; 142: 295–305.
Tseng YH, He TC . Bone morphogenetic proteins and adipocyte differentiation. Cellscience Rev 2007; 3: 342–360.
Rebbapragada A, Benchabane H, Wrana JL, Celeste AJ, Attisano L . Myostatin signals through a transforming growth factor beta-like signaling pathway to block adipogenesis. Mol Cell Biol 2003; 23: 7230–7242.
Rodbell M . Metabolism of isolated fat cells. J Biol Chem 1964; 239: 375–380.
Hino J, Tateyama H, Minamino N, Kangawa K, Matsuo H . Isolation and identification of human brain natriuretic peptides in cardiac atrium. Biochem Biophys Res Commun 1990; 167: 693–700.
Hu E, Liang P, Spiegelman BM . AdipoQ is a novel adipose-specific gene dysregulated in obesity. J Biol Chem 1996; 271: 10697–10703.
Gesta S, Bezy O, Mori MA, Macotela Y, Lee KY, Kahn CR . Mesodermal developmental gene Tbx15 impairs adipocyte differentiation and mitochondrial respiration. Proc Natl Acad Sci USA 2011; 108: 2771–2776.
Guo W, Flanagan J, Jasuja R, Kirkland J, Jiang L, Bhasin S . The effects of myostatin on adipogenic differentiation of human bone marrow-derived mesenchymal stem cells are mediated through cross-communication between Smad3 and Wnt/beta-catenin signaling pathways. J Biol Chem 2008; 283: 9136–9145.
Constam DB, Robertson EJ . Regulation of bone morphogenetic protein activity by pro domains and proprotein convertases. J Cell Biol 1999; 144: 139–149.
Cui YZ, Hackenmiller R, Berg L, Jean F, Nakayama T, Thomas G et al. The activity and signaling range of mature BMP-4 is regulated by sequential cleavage at two sites within the prodomain of the precursor. Genes Dev 2001; 15: 2797–2802.
Hashimoto O, Moore RK, Shimasaki S . Posttranslational processing of mouse and human BMP-15: potential implication in the determination of ovulation quota. Proc Natl Acad Sci USA 2005; 102: 5426–5431.
Shimmi O, Umulis D, Othmer H, O'Connor MB . Facilitated transport of a Dpp/Scw heterodimer by Sog/Tsg leads to robust patterning of the Drosophila blastoderm embryo. Cell 2005; 120: 873–886.
Sopory S, Nelsen SM, Degnin C, Wong C, Christian JL . Regulation of bone morphogenetic protein-4 activity by sequence elements within the prodomain. J Biol Chem 2006; 281: 34021–34031.
Allendorph GP, Isaacs MJ, Kawakami Y, Belmonte JCI, Choe S . BMP-3 and BMP-6 structures illuminate the nature of binding specificity with receptors. Biochemistry 2007; 46: 12238–12247.
Pearsall RS, Canalis E, Cornwall-Brady M, Underwood KW, Haigis B, Ucran J et al. A soluble activin type IIA receptor induces bone formation and improves skeletal integrity. Proc Natl Acad Sci USA 2008; 105: 7082–7087.
Acknowledgements
We thank Dr Shin-ichiro Nishimatsu for characterizing the antibody and CHO/BMP-3b CM and for helpful discussions. We thank Drs Yukari Date and Kazuki Sasaki for evaluating the antibody, and Dr Yuji Arai for the BMP-3b transgenic mice. We appreciate the technical assistance of Ms Michiyo Miyazaki. This study was supported in part by KAKENHI (20591107, 21790900, 21591189, 22126002), the Program for Promotion of Fundamental Studies in Health Science of the National Institute of Biomedical Innovation of Japan and the Takeda Scientific Foundation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on International Journal of Obesity website
Supplementary information
Rights and permissions
This work is licensed under the Creative Commons Attribution-NonCommercial-No Derivative Works 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/3.0/
About this article
Cite this article
Hino, J., Miyazawa, T., Miyazato, M. et al. Bone morphogenetic protein-3b (BMP-3b) is expressed in adipocytes and inhibits adipogenesis as a unique complex. Int J Obes 36, 725–734 (2012). https://doi.org/10.1038/ijo.2011.124
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ijo.2011.124