Abstract
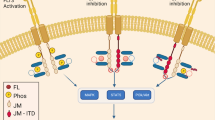
Activating mutations in FLT3 occur in ~30% of adult acute myeloid leukemia, primarily consisting of internal tandem duplication (ITD) mutations (~25%) and point mutations in the tyrosine kinase domain (~5%), commonly at the activation loop residue D835. Secondary kinase domain mutations in FLT3-ITD, particularly at the D835 residue are frequently associated with acquired clinical resistance to effective FLT3 tyrosine kinase inhibitors (TKIs). Molecular docking studies have suggested that D835 mutations primarily confer resistance by stabilizing an active Asp-Phe-Gly in (‘DFG-in’) kinase conformation unfavorable to the binding of type II FLT3 TKIs, which target a ‘DFG-out’ inactive conformation. We profiled the activity of active type II FLT3 TKIs against D835 kinase domain mutants that have been clinically detected to date. We found that type II inhibitors (quizartinib, sorafenib, ponatinib and PLX3397) retain activity against specific D835 substitutions. Modeling studies suggest that bulky hydrophobic substitutions (D835Y/V/I/F) at this residue are particularly resistant, whereas mutations that preserve interactions between D835 and S838 are relatively sensitive (D835E/N). All mutants retain sensitivity to the type I inhibitor crenolanib. These results suggest that patients with relatively sensitive D835 mutations should be included in clinical trials of type II FLT3 TKIs.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Man CH, Fung TK, Ho C, Han HH, Chow HC, Ma AC et al. Sorafenib treatment of FLT3-ITD(+) acute myeloid leukemia: favorable initial outcome and mechanisms of subsequent nonresponsiveness associated with the emergence of a D835 mutation. Blood 2012; 119: 5133–5143.
Tallman MS, Schiller G, Trone D, Gammon G, Goldberg S, Perl AE et al. Results of a phase 2 randomized, open-label, study of lower doses of quizartinib (AC220; ASP2689) in subjects with FLT3-ITD positive relapsed or refractory acute myeloid leukemia (AML). Blood 2013; 122: 494.
Shah NP, Talpaz M, Deininger MW, Mauro MJ, Flinn IW, Bixby D et al. Ponatinib in patients with refractory acute myeloid leukaemia: findings from a phase 1 study. Br J Haematol 2013; 162: 548–552.
Randhawa JK, Kantarjian HM, Borthakur G, Thompson PA, Konopleva M, Daver N et al. Results of a phase II study of crenolanib in relapsed/refractory acute myeloid leukemia patients (Pts) with activating FLT3 mutations. ASH Annual Meeting Abstracts; presented at Annual Meeting of the American Society of Hematology in San Francisco, CA, USA, 2014, vol. 124, pp. 389.
Smith CC, Wang Q, Chin CS, Salerno S, Damon LE, Levis MJ et al. Validation of ITD mutations in FLT3 as a therapeutic target in human acute myeloid leukaemia. Nature 2012; 485: 260–263.
Baker SD, Zimmerman EI, Wang YD, Orwick S, Zatechka DS, Buaboonnam J et al. Emergence of polyclonal FLT3 tyrosine kinase domain mutations during sequential therapy with sorafenib and sunitinib in FLT3-ITD-positive acute myeloid leukemia. Clin Cancer Res 2013; 19: 5758–5768.
Smith CC, Zhang C, Lin K, Lasater EA, Zhang Y, Massi E et al. Characterizing and overriding the structural mechanism of the quizartinib-resistant FLT3 ‘gatekeeper’ F691L mutation with PLX3397. Cancer Discov 2015; 5: 668–679.
Smith CC, Lasater EA, Lin KC, Wang Q, McCreery MQ, Stewart WK et al. Crenolanib is a selective type I pan-FLT3 inhibitor. Proc Natl Acad Sci USA 2014; 111: 5319–5324.
Sali A, Blundell TL . Comparative protein modelling by satisfaction of spatial restraints. J Mol Biol 1993; 234: 779–815.
Griffith J, Black J, Faerman C, Swenson L, Wynn M, Lu F et al. The structural basis for autoinhibition of FLT3 by the juxtamembrane domain. Mol Cell 2004; 13: 169–178.
Dong GQ, Fan H, Schneidman-Duhovny D, Webb B, Sali A . Optimized atomic statistical potentials: assessment of protein interfaces and loops. Bioinformatics 2013; 29: 3158–3166.
Smith CC, Lasater EA, Zhu X, Lin KC, Stewart WK, Damon LE et al. Activity of ponatinib against clinically-relevant AC220-resistant kinase domain mutants of FLT3-ITD. Blood 2013; 121: 3165–3171.
Galanis A, Ma H, Rajkhowa T, Ramachandran A, Small D, Cortes J et al. Crenolanib is a potent inhibitor of FLT3 with activity against resistance-conferring point mutants. Blood 2014; 123: 94–100.
Wodicka LM, Ciceri P, Davis MI, Hunt JP, Floyd M, Salerno S et al. Activation state-dependent binding of small molecule kinase inhibitors: structural insights from biochemistry. Chem Biol 2010; 17: 1241–1249.
Mol CD, Dougan DR, Schneider TR, Skene RJ, Kraus ML, Scheibe DN et al. Structural basis for the autoinhibition and STI-571 inhibition of c-Kit tyrosine kinase. J Biol Chem 2004; 279: 31655–31663.
Fairman R, Shoemaker KR, York EJ, Stewart JM, Baldwin RL . Further studies of the helix dipole model: effects of a free alpha-NH3+ or alpha-COO- group on helix stability. Proteins 1989; 5: 1–7.
Hol WG, van Duijnen PT, Berendsen HJ . The alpha-helix dipole and the properties of proteins. Nature 1978; 273: 443–446.
Acknowledgements
This work was supported by grants from the National Cancer Institute (1R01 CA166616-01) (NPS) and the Leukemia and Lymphoma Society (CCS and NPS). CCS is an ASH Faculty Scholar and recipient of a Hellman Family Foundation Early Career Faculty Award. NPS acknowledges the generous support of Arthur Kern, Mark Maymar and the Edward S Ageno family. The authors thank Evan Massi for technical assistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
NPS has received research funding for the conduct of clinical trials from ARIAD Pharmaceuticals and Ambit Biosciences. NPS has received research funding from Daiichi-Sankyo and Plexxikon Inc. CCS has received research funding for the conduct of clinical trials from Plexxikon Inc. and Astellas Pharma. CCS, KL and A. Stecula designed experiments, performed research, analyzed data and wrote the manuscript. NPS and AS designed experiments, analyzed data and wrote the manuscript.
Additional information
Supplementary Information accompanies this paper on the Leukemia website
Supplementary information
Rights and permissions
About this article
Cite this article
Smith, C., Lin, K., Stecula, A. et al. FLT3 D835 mutations confer differential resistance to type II FLT3 inhibitors. Leukemia 29, 2390–2392 (2015). https://doi.org/10.1038/leu.2015.165
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/leu.2015.165
This article is cited by
-
Characterisation of FLT3 alterations in childhood acute lymphoblastic leukaemia
British Journal of Cancer (2024)
-
Indole-based FLT3 inhibitors and related scaffolds as potential therapeutic agents for acute myeloid leukemia
BMC Chemistry (2023)
-
Recent advances in targeted therapies in acute myeloid leukemia
Journal of Hematology & Oncology (2023)
-
Resistance to targeted therapies in acute myeloid leukemia
Clinical & Experimental Metastasis (2023)
-
A T cell receptor targeting a recurrent driver mutation in FLT3 mediates elimination of primary human acute myeloid leukemia in vivo
Nature Cancer (2023)