Abstract
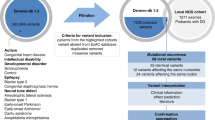
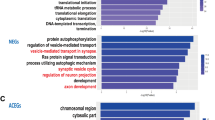
Currently, many studies on neuropsychiatric disorders have utilized massive trio-based whole-exome sequencing (WES) and whole-genome sequencing (WGS) to identify numerous de novo mutations (DNMs). Here, we retrieved 17 104 DNMs from 3555 trios across four neuropsychiatric disorders: autism spectrum disorder, epileptic encephalopathy, intellectual disability and schizophrenia, in addition to unaffected siblings (control), from 36 studies by WES/WGS. After eliminating non-exonic variants, we focused on 3334 exonic DNMs for evaluation of their association with these diseases. Our results revealed a higher prevalence of DNMs in the probands of all four disorders compared with the one in the controls (P<1.3 × 10−7). The elevated DNM frequency is dominated by loss-of-function/deleterious single-nucleotide variants and frameshift indels (that is, extreme mutations, P<4.5 × 10−5). With extensive annotation of these ‘extreme’ mutations, we prioritized 764 candidate genes in these four disorders. A combined analysis of Gene Ontology, microRNA targets and transcription factor targets revealed shared biological process and non-coding regulatory elements of candidate genes in the pathology of neuropsychiatric disorders. In addition, weighted gene co-expression network analysis of human laminar-specific neocortical expression data showed that candidate genes are convergent on eight shared modules with specific layer enrichment and biological process features. Furthermore, we identified that 53 candidate genes are associated with more than one disorder (P<0.000001), suggesting a possibly shared genetic etiology underlying these disorders. Particularly, DNMs of the SCN2A gene are frequently occurred across all four disorders. Finally, we constructed a freely available NPdenovo database, which provides a comprehensive catalog of the DNMs identified in neuropsychiatric disorders.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Bamshad MJ, Ng SB, Bigham AW, Tabor HK, Emond MJ, Nickerson DA et al. Exome sequencing as a tool for Mendelian disease gene discovery. Nat Rev Genet 2011; 12: 745–755.
Goldstein DB, Allen A, Keebler J, Margulies EH, Petrou S, Petrovski S et al. Sequencing studies in human genetics: design and interpretation. Nat Rev Genet 2013; 14: 460–470.
MacArthur D, Manolio T, Dimmock D, Rehm H, Shendure J, Abecasis G et al. Guidelines for investigating causality of sequence variants in human disease. Nature 2014; 508: 469–476.
Ku C, Polychronakos C, Tan E, Naidoo N, Pawitan Y, Roukos D et al. A new paradigm emerges from the study of de novo mutations in the context of neurodevelopmental disease. Mol Psychiatry 2013; 18: 141–153.
Michaelson JJ, Shi Y, Gujral M, Zheng H, Malhotra D, Jin X et al. Whole-genome sequencing in autism identifies hot spots for de novo germline mutation. Cell 2012; 151: 1431–1442.
Yu TW, Chahrour MH, Coulter ME, Jiralerspong S, Okamura-Ikeda K, Ataman B et al. Using whole-exome sequencing to identify inherited causes of autism. Neuron 2013; 77: 259–273.
Hoischen A, Krumm N, Eichler EE . Prioritization of neurodevelopmental disease genes by discovery of new mutations. Nature neuroscience 2014; 17: 764–772.
Krumm N, O'Roak BJ, Shendure J, Eichler EE . A de novo convergence of autism genetics and molecular neuroscience. Trends Neurosci 2014; 37: 95–105.
Epi4K Consortium. De novo mutations in epileptic encephalopathies. Nature 2013; 501: 217–221.
Veltman JA, Brunner HG . De novo mutations in human genetic disease. Nat Rev Genet 2012; 13: 565–575.
Iossifov I, Ronemus M, Levy D, Wang Z, Hakker I, Rosenbaum J et al. De novo gene disruptions in children on the autistic spectrum. Neuron 2012; 74: 285–299.
Neale BM, Kou Y, Liu L, Ma’ayan A, Samocha KE, Sabo A et al. Patterns and rates of exonic de novo mutations in autism spectrum disorders. Nature 2012; 485: 242–245.
O’Roak BJ, Vives L, Girirajan S, Karakoc E, Krumm N, Coe BP et al. Sporadic autism exomes reveal a highly interconnected protein network of de novo mutations. Nature 2012; 485: 246–250.
Sanders SJ, Murtha MT, Gupta AR, Murdoch JD, Raubeson MJ, Willsey AJ et al. De novo mutations revealed by whole-exome sequencing are strongly associated with autism. Nature 2012; 485: 237–241.
Jiang Y-h, Yuen RK, Jin X, Wang M, Chen N, Wu X et al. Detection of clinically relevant genetic variants in autism spectrum disorder by whole-genome sequencing. Am J Hum Genet 2013; 93: 249–263.
de Ligt J, Willemsen MH, van Bon BW, Kleefstra T, Yntema HG, Kroes T et al. Diagnostic exome sequencing in persons with severe intellectual disability. N Engl J Med 2012; 367: 1921–1929.
Rauch A, Wieczorek D, Graf E, Wieland T, Endele S, Schwarzmayr T et al. Range of genetic mutations associated with severe non-syndromic sporadic intellectual disability: an exome sequencing study. Lancet 2012; 380: 1674–1682.
Xu B, Ionita-Laza I, Roos JL, Boone B, Woodrick S, Sun Y et al. De novo gene mutations highlight patterns of genetic and neural complexity in schizophrenia. Nat Genet 2012; 44: 1365–1369.
Fromer M, Pocklington AJ, Kavanagh DH, Williams HJ, Dwyer S, Gormley P et al. De novo mutations in schizophrenia implicate synaptic networks. Nature 2014; 506: 179–184.
Gulsuner S, Walsh T, Watts AC, Lee MK, Thornton AM, Casadei S et al. Spatial and temporal mapping of de novo mutations in schizophrenia to a fetal prefrontal cortical network. Cell 2013; 154: 518–529.
Gratten J, Visscher PM, Mowry BJ, Wray NR . Interpreting the role of de novo protein-coding mutations in neuropsychiatric disease. Nat Genet 2013; 45: 234–238.
Sullivan PF, Daly MJ, O'Donovan M . Genetic architectures of psychiatric disorders: the emerging picture and its implications. Nat Rev Genet 2012; 13: 537–551.
Crow JF . The origins, patterns and implications of human spontaneous mutation. Nat Rev Genet 2000; 1: 40–47.
Eyre-Walker A, Keightley PD . The distribution of fitness effects of new mutations. Nat Rev Genet 2007; 8: 610–618.
Krumm N, O’Roak BJ, Shendure J, Eichler EE . A de novo convergence of autism genetics and molecular neuroscience. Trends Neurosci 2014; 37: 95–105.
Poultney CS, Samocha K, Kou Y, Liu L, Walker S, Singh T et al. Synaptic, transcriptional and chromatin genes disrupted in autism. Nature 2014; 515: 209–215.
Iossifov I, O'Roak BJ, Sanders SJ, Ronemus M, Krumm N, Levy D et al. The contribution of de novo coding mutations to autism spectrum disorder. Nature 2014; 515: 216–221.
Wang K, Li M, Hakonarson H . ANNOVAR: functional annotation of genetic variants from high-throughput sequencing data. Nucleic Acids Res 2010; 38: e164.
Liu X, Jian X, Boerwinkle E . dbNSFP v2. 0: a database of human non-synonymous SNVs and their functional predictions and annotations. Hum Mutat 2013; 34: E2393–E2402.
Kumar P, Henikoff S, Ng PC . Predicting the effects of coding non-synonymous variants on protein function using the SIFT algorithm. Nat Protoc 2009; 4: 1073–1081.
Adzhubei IA, Schmidt S, Peshkin L, Ramensky VE, Gerasimova A, Bork P et al. A method and server for predicting damaging missense mutations. Nat Methods 2010; 7: 248–249.
Schwarz JM, Rödelsperger C, Schuelke M, Seelow D . MutationTaster evaluates disease-causing potential of sequence alterations. Nat Methods 2010; 7: 575–576.
Reva B, Antipin Y, Sander C . Predicting the functional impact of protein mutations: application to cancer genomics. Nucleic Acids Res 2011; 39: e118–e118.
Chun S, Fay JC . Identification of deleterious mutations within three human genomes. Genome Res 2009; 19: 1553–1561.
Shihab HA, Gough J, Cooper DN, Stenson PD, Barker GL, Edwards KJ et al. Predicting the functional, molecular, and phenotypic consequences of amino acid substitutions using hidden Markov models. Hum Mutat 2013; 34: 57–65.
Davydov EV, Goode DL, Sirota M, Cooper GM, Sidow A, Batzoglou S . Identifying a high fraction of the human genome to be under selective constraint using GERP++. PLoS Comput Biol 2010; 6: e1001025.
Pollard KS, Hubisz MJ, Rosenbloom KR, Siepel A . Detection of nonneutral substitution rates on mammalian phylogenies. Genome Res 2010; 20: 110–121.
Garber M, Guttman M, Clamp M, Zody MC, Friedman N, Xie X . Identifying novel constrained elements by exploiting biased substitution patterns. Bioinformatics 2009; 25: i54–i62.
Lindblad-Toh K, Garber M, Zuk O, Lin MF, Parker BJ, Washietl S et al. A high-resolution map of human evolutionary constraint using 29 mammals. Nature 2011; 478: 476–482.
He X, Sanders SJ, Liu L, De Rubeis S, Lim ET, Sutcliffe JS et al. Integrated model of de novo and inherited genetic variants yields greater power to identify risk genes. PLoS Genet 2013; 9: e1003671.
Sunkin SM, Ng L, Lau C, Dolbeare T, Gilbert TL, Thompson CL et al. Allen Brain Atlas: an integrated spatio-temporal portal for exploring the central nervous system. Nucleic Acids Res 2013; 41: D996–D1008.
Miller JA, Ding S-L, Sunkin SM, Smith KA, Ng L, Szafer A et al. Transcriptional landscape of the prenatal human brain. Nature 2014; 508: 199–206.
Wang J, Duncan D, Shi Z, Zhang B., WEB-based GEne SeT . AnaLysis Toolkit (WebGestalt): update 2013. Nucleic Acids Res 2013; 41: W77–W83.
Wenger AM, Clarke SL, Notwell JH, Chung T, Tuteja G, Guturu H et al. The enhancer landscape during early neocortical development reveals patterns of dense regulation and co-option. PLoS Genet 2013; 9: e1003728.
Roshan R, Shridhar S, Sarangdhar MA, Banik A, Chawla M, Garg M et al. Brain-specific knockdown of miR-29 results in neuronal cell death and ataxia in miceRNA 2014; 20: 1287–1297.
Rakic P . Evolution of the neocortex: a perspective from developmental biology. Nat Rev Neurosci 2009; 10: 724–735.
Liao Y, Anttonen A-K, Liukkonen E, Gaily E, Maljevic S, Schubert S et al. SCN2A mutation associated with neonatal epilepsy, late-onset episodic ataxia, myoclonus, and pain. Neurology 2010; 75: 1454–1458.
Lemke JR, Hendrickx R, Geider K, Laube B, Schwake M, Harvey RJ et al. GRIN2B mutations in West syndrome and intellectual disability with focal epilepsy. Ann Neurol 2014; 75: 147–154.
Coe BP, Witherspoon K, Rosenfeld JA, van Bon BW, Vulto-van Silfhout AT, Bosco P et al. Refining analyses of copy number variation identifies specific genes associated with developmental delay. Nat Genet 2014; 46: 1063–1071.
Liu L, Sabo A, Neale BM, Nagaswamy U, Stevens C, Lim E et al. Analysis of rare, exonic variation amongst subjects with autism spectrum disorders and population controls. PLoS Genet 2013; 9: e1003443.
Ben-David E, Shifman S . Combined analysis of exome sequencing points toward a major role for transcription regulation during brain development in autism. Mol Psychiatry 2012; 18: 1054–1056.
Schuurs-Hoeijmakers JH, Oh EC, Vissers LE, Swinkels ME, Gilissen C, Willemsen MA et al. Recurrent de novo mutations in PACS1 cause defective cranial-neural-crest migration and define a recognizable intellectual-disability syndrome. Am J Hum Genet 2012; 91: 1122–1127.
Carvill GL, Heavin SB, Yendle SC, McMahon JM, O'Roak BJ, Cook J et al. Targeted resequencing in epileptic encephalopathies identifies de novo mutations in CHD2 and SYNGAP1. Nat Genet 2013; 45: 825–830.
O'Roak BJ, Vives L, Fu W, Egertson JD, Stanaway IB, Phelps IG et al. Multiplex targeted sequencing identifies recurrently mutated genes in autism spectrum disorders. Science 2012; 338: 1619–1622.
Stessman HA, Bernier R, Eichler EE . A genotype-first approach to defining the subtypes of a complex disease. Cell 2014; 156: 872–877.
Bernier R, Golzio C, Xiong B, Stessman HA, Coe BP, Penn O et al. Disruptive CHD8 mutations define a subtype of autism early in development. Cell 2014; 158: 263–276.
Helsmoortel C, Vulto-van Silfhout AT, Coe BP, Vandeweyer G, Rooms L, van den Ende J et al. A SWI/SNF-related autism syndrome caused by de novo mutations in ADNP. Nat Genet 2014; 46: 380–384.
Albert PR, Vahid-Ansari F, Luckhart C . Serotonin-prefrontal cortical circuitry in anxiety and depression phenotypes: pivotal role of pre-and post-synaptic 5-HT1A receptor expression. Front Behav Neurosci 2014; 8: 199.
Vulto-van Silfhout AT, Rajamanickam S, Jensik PJ, Vergult S, de Rocker N, Newhall KJ et al. Mutations affecting the SAND domain of DEAF1 cause intellectual disability with severe speech impairment and behavioral problems. Am J Hum Genet 2014; 94: 649–661.
Hamilton PJ, Campbell NG, Sharma S, Erreger K, Hansen FH, Saunders C et al. De novo mutation in the dopamine transporter gene associates dopamine dysfunction with autism spectrum disorder. Mol Psychiatry 2013; 18: 1315–1323.
Ebert DH, Greenberg ME . Activity-dependent neuronal signalling and autism spectrum disorder. Nature 2013; 493: 327–337.
Ronemus M, Iossifov I, Levy D, Wigler M . The role of de novo mutations in the genetics of autism spectrum disorders. Nat Rev Genet 2014; 15: 133–141.
Maurano MT, Humbert R, Rynes E, Thurman RE, Haugen E, Wang H et al. Systematic localization of common disease-associated variation in regulatory DNA. Science 2012; 337: 1190–1195.
Khurana E, Fu Y, Colonna V, Mu XJ, Kang HM, Lappalainen T et al. Integrative annotation of variants from 1092 humans: application to cancer genomics. Science 2013; 342: 1235587.
Krichevsky AM, King KS, Donahue CP, Khrapko K, Kosik KS . A microRNA array reveals extensive regulation of microRNAs during brain development. RNA 2003; 9: 1274–1281.
Maffioletti E, Tardito D, Gennarelli M, Bocchio-Chiavetto L . Micro spies from the brain to the periphery: new clues from studies on microRNAs in neuropsychiatric disorders. Front Cell Neurosci 2014; 8: 75.
Xu B, Hsu PK, Karayiorgou M, Gogos JA . MicroRNA dysregulation in neuropsychiatric disorders and cognitive dysfunction. Neurobiol Dis 2012; 46: 291–301.
Parikshak NN, Luo R, Zhang A, Won H, Lowe JK, Chandran V et al. Integrative functional genomic analyses implicate specific molecular pathways and circuits in autism. Cell 2013; 155: 1008–1021.
Willsey AJ, Sanders SJ, Li M, Dong S, Tebbenkamp AT, Muhle RA et al. Coexpression networks implicate human midfetal deep cortical projection neurons in the pathogenesis of autism. Cell 2013; 155: 997–1007.
Hormozdiari F, Penn O, Borenstein E, Eichler E . The discovery of integrated gene networks for autism and related disorders. Genome Res 2014; 25: 142–154, gr. 178855.178114.
Zhu X, Need AC, Petrovski S, Goldstein DB . One gene, many neuropsychiatric disorders: lessons from Mendelian diseases. Nat Neurosci 2014; 17: 773–781.
Cristino A, Williams S, Hawi Z, An J, Bellgrove M, Schwartz C et al. Neurodevelopmental and neuropsychiatric disorders represent an interconnected molecular system. Mol Psychiatry 2013; 19: 294–301.
McCarthy SE, Gillis J, Kramer M, Lihm J, Yoon S, Berstein Y et al. De novo mutations in schizophrenia implicate chromatin remodeling and support a genetic overlap with autism and intellectual disability. Mol Psychiatry 2014; 19: 652–658.
Acknowledgements
The project was funded by the National Natural Science Foundation of China (31171236/C060503), the National Basic Research Program of China (No. 2012CB517902 and 2012CB517904), the National ‘12th Five-Year’ scientific and technological support projects (No. 2012BAI03B02) and the Special Funds of National Health and Family Planning Commission of China (No. 201302002). As a disclaimer, Tao Cai represented his own perspective in the article, not NIDCR/NIH.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Molecular Psychiatry website
Supplementary information
Rights and permissions
About this article
Cite this article
Li, J., Cai, T., Jiang, Y. et al. Genes with de novo mutations are shared by four neuropsychiatric disorders discovered from NPdenovo database. Mol Psychiatry 21, 290–297 (2016). https://doi.org/10.1038/mp.2015.40
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/mp.2015.40
This article is cited by
-
Inversed Effects of Nav1.2 Deficiency at Medial Prefrontal Cortex and Ventral Tegmental Area for Prepulse Inhibition in Acoustic Startle Response
Molecular Neurobiology (2024)
-
The possible role of sodium leakage channel localization factor-1 in the pathophysiology and severity of autism spectrum disorders
Scientific Reports (2023)
-
Targeted sequencing and integrative analysis to prioritize candidate genes in neurodevelopmental disorders
Molecular Neurobiology (2021)
-
SCN2A channelopathies in the autism spectrum of neuropsychiatric disorders: a role for pluripotent stem cells?
Molecular Autism (2020)
-
Functional relationships between recessive inherited genes and genes with de novo variants in autism spectrum disorder
Molecular Autism (2020)