Abstract
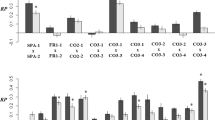
Genetic correlations among traits are important in evolution, as they can constrain evolutionary change or reflect past selection for combinations of traits1,2. Constraints and integration depend on whether the correlations are caused by pleiotropy or linkage disequilibrium3, but these genetic mechanisms underlying correlations remain largely unknown in natural populations4. Quantitative trait locus (QTL) mapping studies do not adequately address the mechanisms of within-population genetic correlations because they rely on crosses between distinct species, inbred lines or selected lines (see ref. 5), and they cannot distinguish moderate linkage disequilibrium from pleiotropy because they commonly rely on only one or two episodes of recombination6. Here I report that after nine generations of enforced random mating (nine episodes of recombination), correlations between six floral traits in wild radish plants are unchanged, showing that pleiotropy generates the correlations. There is no evidence for linkage disequilibrium despite previous correlational selection acting on one functionally integrated pair of traits7. This study provides direct evidence of the genetic mechanisms underlying correlations between quantitative traits in a natural population and suggests that there may be constraints on the independent evolution of pairs of highly correlated traits.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Maynard Smith, J. et al. Developmental constraints and evolution. Quart. Rev. Biol. 60, 265–287 (1985)
Deng, H.-W., Haynatzka, V., Spitze, K. & Haynatzki, G. The determination of genetic covariances and prediction of evolutionary trajectories based on a genetic correlation matrix. Evolution 53, 1592–1599 (1999)
Lynch, M. & Walsh, B. Genetics and Analysis of Quantitative Traits (Sinauer Associates, Sunderland, Massachusetts, 1998)
Clark, A. G. in Genetic Constraints on Adaptive Evolution (ed. Loeschcke, V.) 25–45 (Springer, Berlin, 1987)
Paterson, A. H. et al. Resolution of quantitative traits into Mendelian factors by using a complete linkage map of restriction fragment length polymorphisms. Nature 335, 721–726 (1988)
Kearsey, M. J. & Farquhar, A. G. L. QTL analysis in plants; where are we now? Heredity 80, 137–142 (1998)
Morgan, M. T. & Conner, J. K. Using genetic markers to directly estimate male selection gradients. Evolution 55, 272–281 (2001)
Futuyma, D. J. Evolutionary Biology (Sinauer Associates, Sunderland, Massachusetts, 1998)
Falconer, D. S. & Mackay, T. F. C. Introduction to Quantitative Genetics (Longman, Harlow, 1996)
Mitchell-Olds, T. Pleiotropy causes long-term genetic constraints on life-history evolution in Brassica rapa. Evolution 50, 1849–1858 (1996)
Lande, R. The genetic correlation between characters maintained by selection, linkage and inbreeding. Genet. Res. 44, 309–320 (1984)
Wright, S. Evolution and the Genetics of Populations. Vol. 2. The Theory of Gene Frequencies (Univ. Chicago Press, Chicago, 1969)
Lande, R. The genetic covariance between characters maintained by pleiotropic mutations. Genetics 94, 203–215 (1980)
Nuzhdin, S. V., Dilda, C. & Mackay, F. C. The genetic architecture of selection response: Inferences from fine-scale mapping of bristle number quantitative trait loci in Drosophila melanogaster. Genetics 153, 1317–1331 (1999)
Conner, J. K. & Via, S. Patterns of phenotypic and genetic correlations among morphological and life-history traits in wild radish, Raphanus raphanistrum. Evolution 47, 704–711 (1993)
Conner, J. K. Floral evolution in wild radish: the roles of pollinators, natural selection, and genetic correlations among traits. Int. J. Plant Sci. 158, S108–S120 (1997)
Hill, J. P. & Lord, E. M. Floral development in Arabidopsis thaliana: a comparison of the wild type and the homeotic pistillata mutant. Can. J. Bot. 67, 2922–2936 (1989)
Shabalina, S. A., Yampolsky, L. Y. & Kondrashov, A. S. Rapid decline of fitness in panmictic populations of Drosophila melanogaster maintained under relaxed natural selection. Proc. Natl Acad. Sci. USA 94, 13034–13039 (1997)
Phillips, P. C. & Arnold, S. J. Hierarchical comparison of genetic variance-covariance matrices. I. Using the Flury hierarchy. Evolution 53, 1506–1515 (1999)
Shaw, R. G. The comparison of quantitative genetic parameters between populations. Evolution 45, 143–151 (1991)
Houle, D., Mezey, J. & Galpern, P. Interpretation of the results of common principal components analysis. Evolution 56, 433–440 (2002)
Shaw, F. H., Shaw, R. G., Wilkinson, G. S. & Turelli, M. Changes in genetic variances and covariances: G whiz! Evolution 49, 1260–1267 (1995)
Bulmer, M. G. Linkage disequilibrium and genetic variability. Genet. Res. 23, 281–289 (1974)
Roff, D. A. & Mousseau, T. A. Does natural selection alter genetic architecture? An evaluation of quantitative genetic variation among populations of Allonemobius socius and A. fasciatus. J. Evol. Biol. 12, 361–369 (1999)
Whitlock, M. C., Phillips, P. C., Moore, F. B. G. & Tonsor, S. J. Multiple fitness peaks and epistasis. Annu. Rev. Ecol. System. 26, 601 (1995)
Crow, J. F. & Kimura, M. An Introduction to Population Genetics Theory (Harper and Row, New York, 1970)
SAS Institute SAS/STAT User's Guide, Version 8 (SAS Institute, Cary, North Carolina, 1999)
Willis, J. H., Coyne, J. A. & Kirkpatrick, M. Can one predict the evolution of quantitative characters without genetics? Evolution 45, 441–444 (1991)
Snedecor, G. W. & Cochran, W. G. Statistical Methods (Iowa State Univ. Press, Ames, Iowa, 1989)
Bulmer, M. G. The Mathematical Theory of Quantitative Genetics (Oxford Univ. Press, Oxford, 1985)
Acknowledgements
I thank C. Haun, T. Hickox, S. Kercher, J. Mudd, C. Stewart and many undergraduates for help in the greenhouse; P. Phillips for help with the CPC analysis and for programs; F. Shaw for analyses with Quercus; E. Brodie, B. Charlesworth, J. Fry, D. Houle, R. Lande, M. Lynch, D. Schluter, M. Turelli, S. Via and M. Whitlock for discussion; and A. Agrawal, M. Duffy, J. Fant, J. Kelly, M. Morgan, P. Phillips, H. Sahli, C. Stewart and S. Via for comments on the manuscript. This work was supported by the US National Science Foundation, the University of Illinois and Michigan State University.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The author declares that he has no competing financial interests.
Rights and permissions
About this article
Cite this article
Conner, J. Genetic mechanisms of floral trait correlations in a natural population. Nature 420, 407–410 (2002). https://doi.org/10.1038/nature01105
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nature01105
This article is cited by
-
Chasing genetic correlation breakers to stimulate population resilience to climate change
Scientific Reports (2022)
-
Multivariate selection and the making and breaking of mutational pleiotropy
Evolutionary Ecology (2022)
-
Correlational selection in the age of genomics
Nature Ecology & Evolution (2021)
-
QTL dissection of floral traits in Streptocarpus (Gesneriaceae)
Euphytica (2020)
-
Genetic relationships between anther and stigma traits revealed by QTL analysis in two rice advanced-generation backcross populations
Euphytica (2018)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.