Abstract
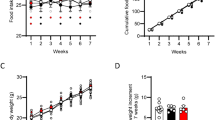
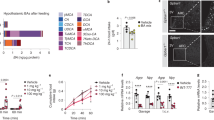
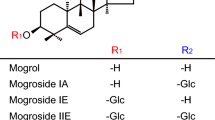
Oleylethanolamide (OEA) is a naturally occurring lipid that regulates satiety and body weight1,2. Although structurally related to the endogenous cannabinoid anandamide, OEA does not bind to cannabinoid receptors and its molecular targets have not been defined. Here we show that OEA binds with high affinity to the peroxisome-proliferator-activated receptor-α (PPAR-α), a nuclear receptor that regulates several aspects of lipid metabolism. Administration of OEA produces satiety and reduces body weight gain in wild-type mice, but not in mice deficient in PPAR-α. Two distinct PPAR-α agonists have similar effects that are also contingent on PPAR-α expression, whereas potent and selective agonists for PPAR-γ and PPAR-β/δ are ineffective. In the small intestine of wild-type but not PPAR-α-null mice, OEA regulates the expression of several PPAR-α target genes: it initiates the transcription of proteins involved in lipid metabolism and represses inducible nitric oxide synthase, an enzyme that may contribute to feeding stimulation. Our results, which show that OEA induces satiety by activating PPAR-α, identify an unexpected role for this nuclear receptor in regulating behaviour, and raise possibilities for the treatment of eating disorders.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Rodríguez de Fonseca, F. et al. An anorexic lipid mediator regulated by feeding. Nature 414, 209–212 (2001)
Gaetani, S., Oveisi, F. & Piomelli, D. Modulation of meal pattern in the rat by the anorexic lipid mediator oleoylethanolamide. Neuropsychopharmacology 28, 1311–1316 (2003)
Göttlicher, M., Widmark, E., Li, Q. & Gustafsson, J. A. Fatty acids activate a chimera of the clofibric acid-activated receptor and the glucocorticoid receptor. Proc. Natl Acad. Sci. USA 89, 4653–4657 (1992)
Kliewer, S. A. et al. Fatty acids and eicosanoids regulate gene expression through direct interactions with peroxisome proliferator-activated receptors α and γ. Proc. Natl Acad. Sci. USA 94, 4318–4323 (1997)
Forman, B. M., Chen, J. & Evans, R. M. Hypolipidemic drugs, polyunsaturated fatty acids, and eicosanoids are ligands for peroxisome proliferator-activated receptors α and δ. Proc. Natl Acad. Sci. USA 94, 4312–4317 (1997)
Desvergne, B. & Wahli, W. Peroxisome proliferator-activated receptors: nuclear control of metabolism. Endocr. Rev. 20, 649–688 (1999)
Chawla, A., Repa, J. J., Evans, R. M. & Mangelsdorf, D. J. Nuclear receptors and lipid physiology: opening the X-files. Science 294, 1866–1870 (2001)
Berger, J. & Moller, D. E. The mechanisms of action of PPARs. Annu. Rev. Med. 53, 409–435 (2002)
Lazennec, G., Canaple, L., Saugy, D. & Wahli, W. Activation of peroxisome proliferator-activated receptors (PPARs) by their ligands and protein kinase A activators. Mol. Endocrinol. 14, 1962–1975 (2000)
Devane, W. A. et al. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science 258, 1946–1949 (1992)
Willson, T. M., Brown, P. J., Sternbach, D. D. & Henke, B. R. The PPARs: from orphan receptors to drug discovery. J. Med. Chem. 43, 527–550 (2000)
Brown, P. J. et al. Chemical compounds as selective activators of PPAR-α. PCT Int. Appl. 32 (2000)
Lee, S. S. et al. Targeted disruption of the α isoform of the peroxisome proliferator-activated receptor gene in mice results in abolishment of the pleiotropic effects of peroxisome proliferators. Mol. Cell. Biol. 15, 3012–3022 (1995)
Butler, A. A. & Cone, R. D. Knockout models resulting in the development of obesity. Trends Genet. 17, S50–S54 (2001)
Oliver, W. R. Jr et al. A selective peroxisome proliferator-activated receptor δ agonist promotes reverse cholesterol transport. Proc. Natl Acad. Sci. USA 98, 5306–5311 (2001)
Chang, A. Y., Wyse, B. M., Gilchrist, B. J., Peterson, T. & Diani, A. R. Ciglitazone, a new hypoglycemic agent. I. Studies in ob/ob and db/db mice, diabetic Chinese hamsters, and normal and streptozotocin-diabetic rats. Diabetes 32, 830–838 (1983)
Martin, G., Schoonjans, K., Lefebvre, A. M., Staels, B. & Auwerx, J. Coordinate regulation of the expression of the fatty acid transport protein and acyl-CoA synthetase genes by PPARα and PPARγ activators. J. Biol. Chem. 272, 28210–28217 (1997)
Escher, P. et al. Rat PPARs: quantitative analysis in adult rat tissues and regulation in fasting and refeeding. Endocrinology 142, 4195–4202 (2001)
Motojima, K., Passilly, P., Peters, J. M., González, F. J. & Latruffe, N. Expression of putative fatty acid transporter genes are regulated by peroxisome proliferator-activated receptor α and γ activators in a tissue- and inducer-specific manner. J. Biol. Chem. 273, 16710–16714 (1998)
Colville-Nash, P. R., Qureshi, S. S. & Willoughby, D. A. Inhibition of inducible nitric oxide synthase by peroxisome proliferator-activated receptor agonist: correlation of induction of heme oxygenase 1. J. Immunol. 161, 978–984 (1998)
Best, J. D. & Jenkins, A. J. Novel agents for managing dyslipidaemia. Expert Opin. Invest. Drugs 10, 1901–1911 (2001)
Cleary, M. P., Kasiske, B., O'Donnell, M. P. & Keane, W. F. Effect of long-term clofibric acid treatment on serum and tissue lipid and cholesterol levels in obese Zucker rats. Atherosclerosis 66, 107–112 (1987)
Chaput, E., Saladin, R., Silvestre, M. & Edgar, A. D. Fenofibrate and rosiglitazone lower serum triglycerides with opposing effects on body weight. Biochem. Biophys. Res. Commun. 271, 445–450 (2000)
Akiyama, T. E. et al. Peroxisome proliferator-activated receptor-α regulates lipid homeostasis, but is not associated with obesity: studies with congenic mouse lines. J. Biol. Chem. 276, 39088–39093 (2001)
Sticker-krongrad, A., Beck, B. & Burlet, C. Nitric oxide mediates hyperphagia of obese Zucker rats: relation to specific changes in the microstructure of feeding behavior. Life Sci. 58, PL9–PL15 (1996)
Janero, D. R. Nutritional aspects of nitric oxide: human health implication and therapeutic opportunities. Nutrition 17, 896–903 (2001)
Chao, E. Y.-H. et al. Thiazoles and oxazole derivatives and their pharmaceutical use. PCT Int. Appl. 83 (2001).
Giuffrida, A., Rodríguez de Fonseca, F. & Piomelli, D. Quantification of bioactive acylethanolamides in rat plasma by electrospray mass spectrometry. Anal. Biochem. 280, 87–93 (2000)
Schmittgen, T. D. et al. Quantitative reverse transcription-polymerase chain reaction to study mRNA decay: comparison of endpoint and real-time methods. Anal. Biochem. 285, 194–204 (2000)
Gregoire, F. M. et al. Diet-induced obesity and hepatic gene expression alterations in C57BL/6J and ICAM-1-deficient mice. Am. J. Physiol. Endocrinol. Metab. 282, E703–E713 (2002)
Acknowledgements
We thank L. Stein for comments; L. Giron, N. Heyrani, N. Izadi and K. Nguyen for help with experiments; M. Guzmán for critically reading the manuscript; and F. Valiño for synthesizing fatty acid ethanolamides. This research was supported by grants (to D.P.) from the National Institute on Drug Abuse. Further support came from the Fondo de Investigación Sanitaria.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
A patent application on this subject been filed on behalf of the University of California Irvine.
Rights and permissions
About this article
Cite this article
Fu, J., Gaetani, S., Oveisi, F. et al. Oleylethanolamide regulates feeding and body weight through activation of the nuclear receptor PPAR-α. Nature 425, 90–93 (2003). https://doi.org/10.1038/nature01921
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nature01921
This article is cited by
-
Evolutionarily related host and microbial pathways regulate fat desaturation in C. elegans
Nature Communications (2024)
-
NAPE-PLD in the ventral tegmental area regulates reward events, feeding and energy homeostasis
Molecular Psychiatry (2024)
-
NAAA-regulated lipid signaling in monocytes controls the induction of hyperalgesic priming in mice
Nature Communications (2024)
-
Inhibition of fatty acid binding protein-5 in the basolateral amygdala induces anxiolytic effects and accelerates fear memory extinction
Psychopharmacology (2024)
-
Oleoylethanolamide facilitates PPARα and TFEB signaling and attenuates Aβ pathology in a mouse model of Alzheimer’s disease
Molecular Neurodegeneration (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.