Abstract
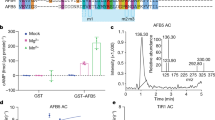
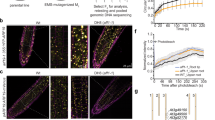
The plant hormone auxin regulates diverse aspects of plant growth and development. Recent studies indicate that auxin acts by promoting the degradation of the Aux/IAA transcriptional repressors through the action of the ubiquitin protein ligase SCFTIR1. The nature of the signalling cascade that leads to this effect is not known. However, recent studies indicate that the auxin receptor and other signalling components involved in this response are soluble factors. Using an in vitro pull-down assay, we demonstrate that the interaction between transport inhibitor response 1 (TIR1) and Aux/IAA proteins does not require stable modification of either protein. Instead auxin promotes the Aux/IAA–SCFTIR1 interaction by binding directly to SCFTIR1. We further show that the loss of TIR1 and three related F-box proteins eliminates saturable auxin binding in plant extracts. Finally, TIR1 synthesized in insect cells binds Aux/IAA proteins in an auxin-dependent manner. Together, these results indicate that TIR1 is an auxin receptor that mediates Aux/IAA degradation and auxin-regulated transcription.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Davies, P. J. in Plant Hormones Physiology, Biochemistry and Molecular Biology (ed. Davies, P. J.) 1–12 (Kluwer Academic, Dordrecht, 1995)
Went, F. W. Wushstoff und wachstum. Rec. Trav. Bot. Neerl. 25, 1–116 (1928)
Weijers, D. & Jurgens, G. Auxin and embryo axis formation: the ends in sight? Curr. Opin. Plant Biol. 8, 32–37 (2005)
Willemsen, V. & Scheres, B. Mechanisms of pattern formation in plant embryogenesis. Annu. Rev. Genet. 38, 587–614 (2004)
Napier, R. M., David, K. M. & Perrot-Rechenmann, C. A short history of auxin-binding proteins. Plant Mol. Biol. 49, 339–348 (2002)
Hagen, G. & Guilfoyle, T. Auxin-responsive gene expression: genes, promoters and regulatory factors. Plant Mol. Biol. 49, 373–385 (2002)
Reed, J. W. Roles and activities of Aux/IAA proteins in Arabidopsis. Trends Plant Sci. 6, 420–425 (2001)
Liscum, E. & Reed, J. W. Genetics of Aux/IAA and ARF action in plant growth and development. Plant Mol. Biol. 49, 387–400 (2002)
Zenser, N., Ellsmore, A., Leasure, C. & Callis, J. Auxin modulates the degradation rate of Aux/IAA proteins. Proc. Natl Acad. Sci. USA 98, 11795–11800 (2001)
Gray, W. M., Kepinski, S., Rouse, D., Leyser, O. & Estelle, M. Auxin regulates SCFTIR1-dependent degradation of Aux/IAA proteins. Nature 414, 271–276 (2001)
Tiwari, S. B., Wang, X. J., Hagen, G. & Guilfoyle, T. J. Aux/IAA proteins are active repressors, and their stability and activity are modulated by auxin. Plant Cell 13, 2809–2822 (2001)
Tian, Q., Nagpal, P. & Reed, J. W. Regulation of Arabidopsis SHY2/IAA3 protein turnover. Plant J. 36, 643–651 (2003)
Ramos, J. A., Zenser, N., Leyser, H. M. & Callis, J. Rapid degradation of Aux/IAA proteins requires conserved amino acids of domain II and is proteasome-dependent. Plant Cell 13, 2349–2360 (2001)
Dharmasiri, N., Dharmasiri, S., Jones, A. M. & Estelle, M. Auxin action in a cell-free system. Curr. Biol. 13, 1418–1422 (2003)
Kepinski, S. & Leyser, O. Auxin-induced SCFTIR1-Aux/IAA interaction involves stable modification of the SCFTIR1 complex. Proc. Natl Acad. Sci. USA 101, 12381–12386 (2004)
Ouellet, F., Overvoorde, P. J. & Theologis, A. IAA17/AXR3. Biochemical insight into an auxin mutant phenotype. Plant Cell 13, 829–842 (2001)
Deshaies, R. J. SCF and Cullin/Ring H2-based ubiquitin ligases. Annu. Rev. Cell Dev. Biol. 15, 435–467 (1999)
Jaakkola, P. et al. Targeting of HIF-alpha to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science 292, 468–472 (2001)
Cardozo, T. & Pagano, M. The SCF ubiquitin ligase: insights into a molecular machine. Nature Rev. Mol. Cell Biol. 5, 739–751 (2004)
Yoshida, Y. et al. E3 ubiquitin ligase that recognizes sugar chains. Nature 418, 438–442 (2002)
Yang, X. et al. The IAA1 protein is encoded by AXR5 and is a substrate of SCFTIR1. Plant J. 40, 772–782 (2004)
Gagne, J. M., Downes, B. P., Shiu, S. H., Durski, A. M. & Vierstra, R. D. The F-box subunit of the SCF E3 complex is encoded by a diverse superfamily of genes in Arabidopsis. Proc. Natl Acad. Sci. USA 99, 11519–11524 (2002)
Gray, W. M. et al. Identification of an SCF ubiquitin-ligase complex required for auxin response in Arabidopsis thaliana. Genes Dev. 13, 1678–1691 (1999)
Xie, D. X., Feys, B. F., James, S., Nieto-Rostro, M. & Turner, J. G. COI1: an Arabidopsis gene required for jasmonate-regulated defense and fertility. Science 280, 1091–1094 (1998)
Kinoshita, T. et al. Binding of brassinosteroids to the extracellular domain of plant receptor kinase BRI1. Nature 433, 167–171 (2005)
Frias, I. et al. A major isoform of the maize plasma membrane H(+ )-ATPase: characterization and induction by auxin in coleoptiles. Plant Cell 8, 1533–1544 (1996)
Blatt, M. R. & Thiel, G. K + channels of stomatal guard cells: bimodal control of the K + inward-rectifier evoked by auxin. Plant J. 5, 55–68 (1994)
Philippar, K. et al. Auxin-induced K + channel expression represents an essential step in coleoptile growth and gravitropism. Proc. Natl Acad. Sci. USA 96, 12186–12191 (1999)
Luthen, H., Claussen, M. & Bottger, M. Growth: progress in auxin research. Prog. Bot. 60, 315–340 (1999)
Pickart, C. M. Mechanisms underlying ubiquitination. Annu. Rev. Biochem. 70, 503–533 (2001)
Acknowledgements
We thank R. Dumas for technical assistance and C. Leblanc for help with insect cell culture. In addition, we thank S. Kepinski and O. Leyser for discussions. Research in the authors' laboratory is supported by grants from the NIH, the NSF and the DOE.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Reprints and permissions information is available at npg.nature.com/reprintsandpermissions. The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Dharmasiri, N., Dharmasiri, S. & Estelle, M. The F-box protein TIR1 is an auxin receptor. Nature 435, 441–445 (2005). https://doi.org/10.1038/nature03543
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nature03543
This article is cited by
-
3,4-Dichlorophenylacetic acid acts as an auxin analog and induces beneficial effects in various crops
Communications Biology (2024)
-
A New In Vitro Growth System for Phenotypic Characterization and Seed Propagation of Arabidopsis thaliana
Journal of Plant Growth Regulation (2024)
-
Different evolutionary patterns of TIR1/AFBs and AUX/IAAs and their implications for the morphogenesis of land plants
BMC Plant Biology (2023)
-
NLR surveillance of pathogen interference with hormone receptors induces immunity
Nature (2023)
-
Insights of auxin signaling F-box genes in wheat (Triticum aestivum L.) and their dynamic expression during the leaf rust infection
Protoplasma (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.