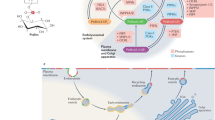
Abstract
Phosphatidylinositol 4,5-bisphosphate (PIP2), which comprises only about 1% of the phospholipids in the cytoplasmic leaflet of the plasma membrane, is the source of three second messengers, activates many ion channels and enzymes, is involved in both endocytosis and exocytosis, anchors proteins to the membrane through several structured domains and has other roles. How can a single lipid in a fluid bilayer regulate so many distinct physiological processes? Spatial organization might be the key to this. Recent studies suggest that membrane proteins concentrate PIP2 and, in response to local increases in intracellular calcium concentration, release it to interact with other biologically important molecules.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Berridge, M. J. & Irvine, R. F. Inositol trisphosphate, a novel second messenger in cellular signal transduction. Nature 312, 315–321 (1984).
Berridge, M. J. & Irvine, R. F. Inositol phosphates and cell signalling. Nature 341, 197–205 (1989).
Rhee, S. G. Regulation of phosphoinositide-specific phospholipase C. Annu. Rev. Biochem. 70, 281–312 (2001).
Rebecchi, M. J. & Pentyala, S. N. Structure, function, and control of phosphoinositide-specific phospholipase C. Physiol. Rev. 80, 1291–1335 (2000).
Swann, K., Larman, M. G., Saunders, C. M. & Lai, F. A. The cytosolic sperm factor that triggers Ca2+ oscillations and egg activation in mammals is a novel phospholipase C: PLCQ. Reproduction 127, 431–439 (2004).
Cantley, L. C. The phosphoinositide 3-kinase pathway. Science 296, 1655–1657 (2002).
Cho, W. & Stahelin, R. V. Membrane-protein interactions in cell signaling and membrane trafficking. Annu. Rev. Biophys. Biomol. Struct. 34, 119–151 (2005).
DiNitto, J. P., Cronin, T. C. & Lambright, D. G. Membrane recognition and targeting by lipid-binding domains. Sci. STKE 2003, re16 (2003).
Lemmon, M. A. Phosphoinositide recognition domains. Traffic 4, 201–213 (2003).
Hurley, J. H. & Meyer, T. Subcellular targeting by membrane lipids. Curr. Opin. Cell Biol. 13, 146–152 (2001).
Suh, B. C. & Hille, B. Regulation of ion channels by phosphatidylinositol 4,5-bisphosphate. Curr. Opin. Neurobiol. 15, 370–378 (2005).
Hilgemann, D. W. Oily barbarians breach ion channel gates. Science 304, 223–224 (2004).
Honing, S. et al. Phosphatidylinositol-(4,5)-bisphosphate regulates sorting signal recognition by the clathrin-associated adaptor complex AP2. Mol. Cell 18, 519–531 (2005).
Wenk, M. R. & De Camilli, P. Protein-lipid interactions and phosphoinositide metabolism in membrane traffic: insights from vesicle recycling in nerve terminals. Proc. Natl Acad. Sci. USA 101, 8262–8269 (2004).
Simonsen, A., Wurmser, A. E., Emr, S. D. & Stenmark, H. The role of phosphoinositides in membrane transport. Curr. Opin. Cell Biol. 13, 485–492 (2001).
Botelho, R. J. et al. Localized biphasic changes in phosphatidylinositol-4,5-bisphosphate at sites of phagocytosis. J. Cell Biol. 151, 1353–1368 (2000).
Holz, R. W. et al. A pleckstrin homology domain specific for phosphatidylinositol 4, 5-bisphosphate (PtdIns-4,5-P2) and fused to green fluorescent protein identifies plasma membrane PtdIns-4,5-P2 as being important in exocytosis. J. Biol. Chem. 275, 17878–17885 (2000).
Martin, T. F. PI(4,5)P2 regulation of surface membrane traffic. Curr. Opin. Cell Biol. 13, 493–499 (2001).
Di Paolo, G. et al. Impaired PtdIns(4,5)P2 synthesis in nerve terminals produces defects in synaptic vesicle trafficking. Nature 431, 415–422 (2004).
Janmey, P. A. & Lindberg, U. Cytoskeletal regulation: rich in lipids. Nature Rev. Mol. Cell Biol. 5, 658–666 (2004).
Papayannopoulos, V. et al. A polybasic motif allows N-WASP to act as a sensor of PIP2 density. Mol. Cell 17, 181–191 (2005).
Raucher, D. et al. Phosphatidylinositol 4,5-bisphosphate functions as a second messenger that regulates cytoskeleton-plasma membrane adhesion. Cell 100, 221–228 (2000).
Sciorra, V. A. et al. Dual role for phosphoinositides in regulation of yeast and mammalian phospholipase D enzymes. J. Cell Biol. 159, 1039–1049 (2002).
Hinchliffe, K. A., Ciruela, A. & Irvine, R. F. PIPkins, their substrates and their products: new functions for old enzymes. Biochim. Biophys. Acta 1436, 87–104 (1998).
van Rheenen, J., Achame, E. M., Janssen, H., Calafat, J. & Jalink, K. PIP2 signaling in lipid domains: a critical re-evaluation. EMBO J. 24, 1664–1673 (2005).
Golub, T. & Caroni, P. PI(4,5)P2-dependent microdomain assemblies capture microtubules to promote and control leading edge motility. J. Cell Biol. 169, 151–165 (2005).
Berridge, M. J., Bootman, M. D. & Roderick, H. L. Calcium signalling: dynamics, homeostasis and remodelling. Nature Rev. Mol. Cell Biol. 4, 517–529 (2003).
Laux, T. et al. GAP43, MARCKS, and CAP23 modulate PI(4,5)P2 at plasmalemmal rafts, and regulate cell cortex actin dynamics through a common mechanism. J. Cell Biol. 149, 1455–1472 (2000).
Dunker, A. K. et al. Intrinsically disordered protein. J. Mol. Graph. Model. 19, 26–59 (2001).
Uversky, V. N. What does it mean to be natively unfolded? Eur. J. Biochem. 269, 2–12 (2002).
Aderem Aderem, A. The MARCKS brothers: a family of protein kinase C substrates. Cell 71, 713–716 (1992).
Blackshear Blackshear, P. J. The MARCKS family of cellular protein kinase C substrates. J. Biol. Chem. 268, 1501–1504 (1993).
McLaughlin, S. & Aderem, A. The myristoyl-electrostatic switch: a modulator of reversible protein-membrane interactions. Trends Biochem. Sci. 20, 272–276 (1995).
Arbuzova, A., Schmitz, A. A. & Vergeres, G. Cross-talk unfolded: MARCKS proteins. Biochem. J. 362, 1–12 (2002).
Sundaram, M., Cook, H. W. & Byers, D. M. The MARCKS family of phospholipid binding proteins: regulation of phospholipase D and other cellular components. Biochem. Cell Biol. 82, 191–200 (2004).
Taniguchi, H. & Manenti, S. Interaction of myristoylated alanine-rich protein kinase C substrate (MARCKS) with membrane phospholipids. J. Biol. Chem. 268, 9960–9963 (1993).
Kim, J., Shishido, T., Jiang, X., Aderem, A. & McLaughlin, S. Phosphorylation, high ionic strength, and calmodulin reverse the binding of MARCKS to phospholipid vesicles. J. Biol. Chem. 269, 28214–28219 (1994).
Rusu, L., Gambhir, A., McLaughlin, S. & Radler, J. Fluorescence correlation spectroscopy studies of peptide and protein binding to phospholipid vesicles. Biophys. J. 87, 1044–1053 (2004).
Gambhir, A. et al. Electrostatic sequestration of PIP2 on phospholipid membranes by basic/aromatic regions of proteins. Biophys. J. 86, 2188–2207 (2004).
Arbuzova, A., Murray, D. & McLaughlin, S. MARCKS, membranes, and calmodulin: kinetics of their interaction. Biochim. Biophys. Acta 1376, 369–379 (1998).
Ohmori, S. et al. Importance of protein kinase C targeting for the phosphorylation of its substrate, myristoylated alanine-rich C-kinase substrate. J. Biol. Chem. 275, 26449–26457 (2000).
Qin, Z. & Cafiso, D. S. Membrane structure of protein kinase C and calmodulin binding domain of myristoylated alanine rich C kinase substrate determined by site-directed spin labeling. Biochemistry 35, 2917–2925 (1996).
Wang, J., Arbuzova, A., Hangyas-Mihalyne, G. & McLaughlin, S. The effector domain of myristoylated alanine-rich C kinase substrate binds strongly to phosphatidylinositol 4,5-bisphosphate. J. Biol. Chem. 276, 5012–5019 (2001).
Victor, K., Jacob, J. & Cafiso, D. S. Interactions controlling the membrane binding of basic protein domains: phenylalanine and the attachment of the myristoylated alanine-rich C-kinase substrate protein to interfaces. Biochemistry 38, 12527–12536 (1999).
Zhang, W., Crocker, E., McLaughlin, S. & Smith, S. O. Binding of peptides with basic and aromatic residues to bilayer membranes: phenylalanine in the myristoylated alanine-rich C kinase substrate effector domain penetrates into the hydrophobic core of the bilayer. J. Biol. Chem. 278, 21459–21466 (2003).
Ellena, J. F., Burnitz, M. C. & Cafiso, D. S. Location of the myristoylated alanine-rich C-kinase substrate (MARCKS) effector domain in negatively charged phospholipid bicelles. Biophys. J. 85, 2442–2448 (2003).
White, S. H. & Wimley, W. C. Membrane protein folding and stability: physical principles. Annu. Rev. Biophys. Biomol. Struct. 28, 319–365 (1999).
Clapperton, J. A., Martin, S. R., Smerdon, S. J., Gamblin, S. J. & Bayley, P. M. Structure of the complex of calmodulin with the target sequence of calmodulin-dependent protein kinase I: studies of the kinase activation mechanism. Biochemistry 41, 14669–14679 (2002).
Porumb, T., Crivici, A., Blackshear, P. J. & Ikura, M. Calcium binding and conformational properties of calmodulin complexed with peptides derived from myristoylated alanine-rich C kinase substrate (MARCKS) and MARCKS-related protein (MRP). Eur. Biophys. J. 25, 239–247 (1997).
Yamauchi, E., Nakatsu, T., Matsubara, M., Kato, H. & Taniguchi, H. Crystal structure of a MARCKS peptide containing the calmodulin-binding domain in complex with Ca2+-calmodulin. Nature Struct. Biol. 10, 226–231 (2003).
Benowitz, L. I. & Routtenberg, A. GAP-43: an intrinsic determinant of neuronal development and plasticity. Trends Neurosci. 20, 84–91 (1997).
Liu, Y. C. & Storm, D. R. Regulation of free calmodulin levels by neuromodulin: neuron growth and regeneration. Trends Pharmacol. Sci. 11, 107–111 (1990).
Liang, X., Lu, Y., Neubert, T. A. & Resh, M. D. Mass spectrometric analysis of GAP-43/neuromodulin reveals the presence of a variety of fatty acylated species. J. Biol. Chem. 277, 33032–33040 (2002).
Skene, J. H. & Virag, I. Posttranslational membrane attachment and dynamic fatty acylation of a neuronal growth cone protein, GAP-43. J. Cell Biol. 108, 613–624 (1989).
Persechini, A. & Stemmer, P. M. Calmodulin is a limiting factor in the cell. Trends Cardiovasc. Med. 12, 3–37 (2002).
Black, D. J., Tran, Q. K. & Persechini, A. Monitoring the total available calmodulin concentration in intact cells over the physiological range in free Ca2+. Cell Calcium 35, 415–425 (2004).
Isotani, E. et al. Real-time evaluation of myosin light chain kinase activation in smooth muscle tissues from a transgenic calmodulin-biosensor mouse. Proc. Natl Acad. Sci. USA 101, 6279–6284 (2004).
Wang, J. et al. Lateral sequestration of phosphatidylinositol 4,5-bisphosphate by the basic effector domain of myristoylated alanine-rich C kinase substrate is due to nonspecific electrostatic interactions. J. Biol. Chem. 277, 34401–34412 (2002).
Holthuis, J. C. & Levine, T. P. Lipid traffic: floppy drives and a superhighway. Nature Rev. Mol. Cell Biol. 6, 209–220 (2005).
McLaughlin, S., Wang, J., Gambhir, A. & Murray, D. PIP2 and proteins: interactions, organization, and information flow. Annu. Rev. Biophys. Biomol. Struct. 31, 151–175 (2002).
McLaughlin, S. The electrostatic properties of membranes. Annu. Rev. Biophys. Biophys. Chem. 18, 113–136 (1989).
Ben-Tal, N., Honig, B., Peitzsch, R. M., Denisov, G. & McLaughlin, S. Binding of small basic peptides to membranes containing acidic lipids: theoretical models and experimental results. Biophys. J. 71, 561–575 (1996).
Murray, D., Arbuzova, A., Honig, B. & McLaughlin, S. The role of electrostatic and nonpolar interactions in the association of peripheral proteins with membranes. Curr. Top. Membr. 52, 271–302 (2002).
Wang, J., Gambhir, A., McLaughlin, S. & Murray, D. A computational model for the electrostatic sequestration of PI(4,5)P2 by membrane-adsorbed basic peptides. Biophys. J. 86, 1969–1986 (2004).
McLaughlin, S., Smith, S. O., Hayman, M. J. & Murray, D. An electrostatic engine model for autoinhibition and activation of the epidermal growth factor receptor (EGFR/ErbB) family. J. Gen. Physiol. 126, 41–53 (2005).
Wollmuth, L. P. & Sobolevsky, A. I. Structure and gating of the glutamate receptor ion channel. Trends Neurosci. 27, 321–328 (2004).
Ehlers, M. D., Zhang, S., Bernhadt, J. P. & Huganir, R. L. Inactivation of NMDA receptors by direct interaction of calmodulin with the NR1 subunit. Cell 84, 745–755 (1996).
Ferguson, K. M. Active and inactive conformations of the epidermal growth factor receptor. Biochem. Soc. Trans. 32, 742–745 (2004).
Uyemura, T., Takagi, H., Yanagida, T. & Sako, Y. Single-molecule analysis of epidermal growth factor signaling that leads to ultrasensitive calcium response. Biophys. J. 88, 3720–3730 (2005).
De Matteis, M. A. & Godi, A. PI-loting membrane traffic. Nature Cell Biol. 6, 487–492 (2004).
Honda, A. et al. Phosphatidylinositol 4-phosphate 5-kinase 4 is a downstream effector of the small G protein ARF6 in membrane ruffle formation. Cell 99, 521–532 (1999).
Tall, E. G., Spector, I., Pentyala, S. N., Bitter, I. & Rebecchi, M. J. Dynamics of phosphatidylinositol 4,5-bisphosphate in actin-rich structures. Curr. Biol. 10, 743–746 (2000).
Watt, S. A., Kular, G., Fleming, I. N., Downes, C. P. & Lucocq, J. M. Subcellular localization of phosphatidylinositol 4,5-bisphosphate using the pleckstrin homology domain of phospholipase C δ1. Biochem. J. 363, 657–666 (2002).
van Rheenen, J. & Jalink, K. Agonist-induced PIP2 hydrolysis inhibits cortical actin dynamics: regulation at a global but not at a micrometer scale. Mol. Biol. Cell 13, 3257–3267 (2002).
Doughman, R. L., Firestone, A. J. & Anderson, R. A. Phosphatidylinositol phosphate kinases put PI4,5P2 in its place. J. Membr. Biol. 194, 77–89 (2003).
Chakravarthy, B., Morley, P. & Whitfield, J. Ca2+-calmodulin and protein kinase Cs: a hypothetical synthesis of their conflicting convergences on shared substrate domains. Trends Neurosci. 22, 12–16 (1999).
Van Haastert, P. J. & Devreotes, P. N. Chemotaxis: signalling the way forward. Nature Rev. Mol. Cell Biol. 5, 626–634 (2004).
Singh, S. M. & Murray, D. Molecular modeling of the membrane targeting of phospholipase C pleckstrin homology domains. Protein Sci. 12, 1934–1953 (2003).
Gallagher, K. & Sharp, K. Electrostatic contributions to heat capacity changes of DNA-ligand binding. Biophys. J. 75, 769–776 (1998).
Nicholls, A., Sharp, K. A. & Honig, B. Protein folding and association: insights from the interfacial and thermodynamic properties of hydrocarbons. Proteins 11, 281–296 (1991).
Hunter, T., Ling, N. & Cooper, J. A. Protein kinase C phosphorylation of the EGF receptor at a threonine residue close to the cytoplasmic face of the plasma membrane. Nature 311, 480–483 (1984).
Ullrich, A. et al. Human epidermal growth factor receptor cDNA sequence and aberrant expression of the amplified gene in A431 epidermoid carcinoma cells. Nature 309, 418–425 (1984).
Li, H., Ruano, M. J. & Villalobo, A. Endogenous calmodulin interacts with the epidermal growth factor receptor in living cells. FEBS Lett. 559, 175–180 (2004).
Graff, J. M., Young, T. N., Johnson, J. D. & Blackshear, P. J. Phosphorylation-regulated calmodulin binding to a prominent cellular substrate for protein kinase C. J. Biol. Chem. 264, 21818–21823 (1989).
Yamniuk, A. P. & Vogel, H. J. Calmodulin’s flexibility allows for promiscuity in its interactions with target proteins and peptides. Mol. Biotechnol. 27, 33–57 (2004).
Acknowledgements
This work was supported by grants from the NIH and Carol M. Baldwin Foundation (to S.M) and NSF (to D.M.).
Author information
Authors and Affiliations
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Additional information
Author Information Reprints and permissions information is available at npg.nature.com/reprintsandpermissions.
Rights and permissions
About this article
Cite this article
McLaughlin, S., Murray, D. Plasma membrane phosphoinositide organization by protein electrostatics. Nature 438, 605–611 (2005). https://doi.org/10.1038/nature04398
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature04398
This article is cited by
-
Critical contributions of pre-S1 shoulder and distal TRP box in DAG-activated TRPC6 channel by PIP2 regulation
Scientific Reports (2022)
-
Spatiotemporal dynamics of membrane surface charge regulates cell polarity and migration
Nature Cell Biology (2022)
-
Phosphatidylinositol 4,5-bisphosphate (PIP2) facilitates norepinephrine transporter dimerization and modulates substrate efflux
Communications Biology (2022)
-
A network of phosphatidylinositol (4,5)-bisphosphate (PIP2) binding sites on the dopamine transporter regulates amphetamine behavior in Drosophila Melanogaster
Molecular Psychiatry (2021)
-
The distribution of phosphatidylinositol 4,5-bisphosphate in the budding yeast plasma membrane
Histochemistry and Cell Biology (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.