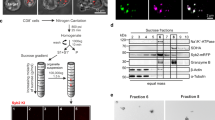
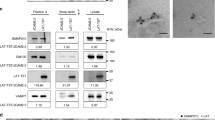
Abstract
Cytotoxic T lymphocytes (CTLs) destroy virally infected and tumorigenic cells by releasing the contents of specialized secretory lysosomes—termed ‘lytic granules’—at the immunological synapse formed between the CTL and the target1. On contact with the target cell, the microtubule organizing centre of the CTL polarizes towards the target2,3 and granules move along microtubules in a minus-end direction towards the polarized microtubule organizing centre. However, the final steps of secretion have remained unclear. Here we show that CTLs do not require actin or plus-end microtubule motors for secretion, but instead the centrosome moves to and contacts the plasma membrane at the central supramolecular activation cluster of the immunological synapse. Actin and IQGAP1 are cleared away from the synapse, and granules are delivered directly to the plasma membrane. These data show that CTLs use a previously unreported mechanism for delivering secretory granules to the immunological synapse, with granule secretion controlled by centrosome delivery to the plasma membrane.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Stinchcombe, J., Bossi, G. & Griffiths, G. M. Linking albinism and immunity: the secrets of secretory lysosomes. Science 305, 55–59 (2004)
Geiger, B., Rosen, D. & Berke, G. Spatial relationships of microtubule-organizing centers and the contact area of cytotoxic T lymphocytes and target cells. J. Cell Biol. 95, 137–143 (1982)
Kupfer, A. & Dennert, G. Reorientation of the microtubule-organizing center and the Golgi apparatus in cloned cytotoxic lymphocytes triggered by binding to lysable target cells. J. Immunol. 133, 2762–2766 (1984)
Stinchcombe, J. C., Bossi, G., Booth, S. & Griffiths, G. M. The immunological synapse of CTL contains a secretory domain and membrane bridges. Immunity 15, 751–761 (2001)
Monks, C. R., Freiberg, B. A., Kupfer, H., Sciaky, N. & Kupfer, A. Three-dimensional segregation of supramolecular activation clusters in T cells. Nature 395, 82–86 (1998)
Lin, J., Miller, M. J. & Shaw, A. S. The c-SMAC: sorting it all out (or in). J. Cell Biol. 170, 177–182 (2005)
Kuhn, J. R. & Poenie, M. Dynamic polarization of the microtubule cytoskeleton during CTL-mediated killing. Immunity 16, 111–121 (2002)
Poenie, M., Kuhn, J. & Combs, J. Real-time visualization of the cytoskeleton and effector functions in T cells. Curr. Opin. Immunol. 16, 428–438 (2004)
Jordens, I., Marsman, M., Kuijl, C. & Neefjes, J. Rab proteins, connecting transport and vesicle fusion. Traffic 6, 1070–1077 (2005)
Jordens, I. et al. The Rab7 effector protein RILP controls lysosomal transport by inducing the recruitment of dynein–dynactin motors. Curr. Biol. 11, 1680–1685 (2001)
Allan, V. J., Thompson, H. M. & McNiven, M. A. Motoring around the Golgi. Nature Cell Biol. 4, E236–E242 (2002)
Cannon, J. L. & Burkhardt, J. K. The regulation of actin remodeling during T-cell–APC conjugate formation. Immunol. Rev. 186, 90–99 (2002)
Stowers, L., Yelon, D., Berg, L. J. & Chant, J. Regulation of the polarization of T cells toward antigen-presenting cells by Ras-related GTPase CDC42. Proc. Natl Acad. Sci. USA 92, 5027–5031 (1995)
Fukata, M. et al. Rac1 and Cdc42 capture microtubules through IQGAP1 and CLIP-170. Cell 109, 873–885 (2002)
Lansbergen, G. & Akhmanova, A. Microtubule plus end: a hub of cellular activities. Traffic 7, 499–507 (2006)
Piel, M., Nordberg, J., Euteneuer, U. & Bornens, M. Centrosome-dependent exit of cytokinesis in animal cells. Science 291, 1550–1553 (2001)
Gromley, A. et al. Centriolin anchoring of exocyst and SNARE complexes at the midbody is required for secretory-vesicle-mediated abscission. Cell 123, 75–87 (2005)
Cowan, C. R. & Hyman, A. A. Centrosomes direct cell polarity independently of microtubule assembly in C. elegans embryos. Nature 431, 92–96 (2004)
Sorokin, S. P. Reconstructions of centriole formation and ciliogenesis in mammalian lungs. J. Cell Sci. 3, 207–230 (1968)
Bornens, M. Centrosome composition and microtubule anchoring mechanisms. Curr. Opin. Cell Biol. 14, 25–34 (2002)
Lange, B. M. & Gull, K. A molecular marker for centriole maturation in the mammalian cell cycle. J. Cell Biol. 130, 919–927 (1995)
Ishikawa, H., Kubo, A., Tsukita, S. & Tsukita, S. Odf2-deficient mother centrioles lack distal/subdistal appendages and the ability to generate primary cilia. Nature Cell Biol. 7, 517–524 (2005)
Fruth, U. et al. A novel serine proteinase (HuTSP) isolated from a cloned human CD8+ cytolytic T-cell line is expressed and secreted by activated CD4+ and CD8+ lymphocytes. Eur. J. Immunol. 17, 1625–1633 (1987)
Acknowledgements
We thank J. Neefjes for the RILP complementary DNA, B. Imhof for actin–GFP, A. Thrasher for lentiviral vectors, M. Simon for anti-granzyme A antibodies, and K. Gull for anti-cenexin antibodies. J. Kaufman, P. A. van der Merwe and K. Gull provided many helpful discussions. This research was funded by the Wellcome Trust. Funding for the Dunn School Bio-imaging facility was provided by the Edward Abraham and Wellcome Trusts. G.M.G. is the recipient of a Royal Society Wolfson Research Merit Award. Author Contributions J.C.S. carried out all experiments except for RILP analysis (G.B.), tomographic reconstruction (E.M. and S.F.) and CTL culture (G.M.G.). J.C.S. and G.M.G. analysed the data and wrote the manuscript with contributions from all authors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Reprints and permissions information is available at www.nature.com/reprints. The authors declare no competing financial interests.
Supplementary information
Supplementary Information guide
This file contains Supplementary Methods, Supplementary Figure 1 and Supplementary Movie Legends 1–10. (DOC 659 kb)
Supplementary Movie 1A
3D reconstruction, rotated around the yz axis, of serial confocal images through a CTL conjugated to a target cell showing CTL centrioles (red) polarized right up to the plasma membrane at the contact site between the CTL and its target (shown in green) during killing of the target cell. (MOV 1358 kb)
Supplementary Movie 1B
3D reconstruction, rotated around the xz axis, of serial confocal images through a CTL conjugated to a target cell showing CTL centrioles (red) polarised right up to the plasma membrane at the contact site between the CTL and its target (shown in green) during killing of the target cell. (MOV 1213 kb)
Supplementary Movie 2
Tomogram generated from a -65-+65° tilt series of an EM section across the contact site between a CTL and its target showing polarisation of the CTL centrosome to the membrane and distribution of the lytic granules, loaded with an electron dense reaction product, at the contact site during target cell killing (AVI 682 kb)
Supplementary Movie 3
Model generated from the tomogram shown in S2 highlighting the distribution of the polarised centrosome (blue), lytic granules (grey), microtubules (red), Golgi complex (green) in the CTL at the contact site. (AVI 1540 kb)
Supplementary Movie 4A
3D reconstruction, rotated around the yz axis, of serial confocal images taken through a CTL conjugated to a target cell showing the CTL centrosome (blue) contacts the plasma membrane within the pSMAC (green) with the leading centriole on the edge of the cSMAC (red). (MOV 854 kb)
Supplementary Movie 4B
3D reconstruction, rotated around the xz axis, of serial confocal images taken through a CTL conjugated to a target cell showing the CTL centrosome (blue) contacts the plasma membrane within the pSMAC (green) with the leading centriole on the edge of the cSMAC (red). (MOV 734 kb)
Supplementary Movie 5A
3D reconstruction, rotated around the yz axis, of serial confocal images through a CTL conjugated to a target cell showing the CTL centrosome (blue) contacts the plasma membrane at the boundary between the cSMAC (red) and the site of lytic granule (green) docking and secretion. (MOV 696 kb)
Supplementary Movie 5B
3D reconstruction, rotated around the xz axis, of serial confocal images through a CTL conjugated to a target cell showing the CTL centrosome (blue) contacts the plasma membrane at the boundary between the cSMAC (red) and the site of lytic granule (green) docking and secretion. (MOV 763 kb)
Supplementary Movie 6
3D reconstruction, rotated around the yx axis, of serial confocal images through a single CTL showing the steady state distribution of IQGAP1 (red), actin (blue) and CD11a (green) in a CTL before it encounters a target cell. (MOV 3333 kb)
Supplementary Movie 7
3D reconstruction, rotated around the yz axis, of serial confocal images through a CTL conjugated to a target cell showing IQGAP1 (red) and actin (blue) cleared from the contact site to outside the pSMAC (CD11a, green) on encounter with a target cell and formation of an immunological synapse. (MOV 4500 kb)
Supplementary Movie 8
3D reconstruction, rotated around the yz axis, of serial confocal images through a CTL conjugated to a target cell showing IQGAP1 (red) present across the whole contact site area in a conjugate where the CTL centrosome (blue) is not polarised and CD11a (green) is not organised into a pSMAC. (MOV 3642 kb)
Supplementary Movie 9
3D reconstruction, rotated around the yz axis, of serial confocal images through a CTL conjugated to a target cell showing IQGAP1 (red) cleared from the contact site in a conjugate where the CTL centrosome (blue) has polarised to the plasma membrane and CD11a (green) is organised into a pSMAC. (MOV 3543 kb)
Supplementary Movie 10
3D reconstruction, rotated around the yz axis, of serial confocal images through a CTL conjugated to a target cell showing IQGAP1 (green) and actin (blue) cleared from the contact site during docking of lytic granules (red) and targeted secretion at the secretory domain of the immunological synapse. (MOV 6704 kb)
Rights and permissions
About this article
Cite this article
Stinchcombe, J., Majorovits, E., Bossi, G. et al. Centrosome polarization delivers secretory granules to the immunological synapse. Nature 443, 462–465 (2006). https://doi.org/10.1038/nature05071
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nature05071
This article is cited by
-
Partial loss of Sorting Nexin 27 resembles age- and Down syndrome-associated T cell dysfunctions
Immunity & Ageing (2024)
-
Extended-Synaptotagmin-1 and -2 control T cell signaling and function
EMBO Reports (2023)
-
The centrosomal protein 131 participates in the regulation of mitochondrial apoptosis
Communications Biology (2023)
-
Tumors evade immune cytotoxicity by altering the surface topology of NK cells
Nature Immunology (2023)
-
Who wins the combat, CAR or TCR?
Leukemia (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.