Abstract
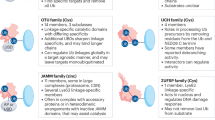
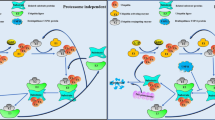
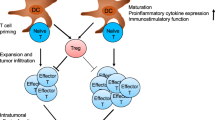
The ubiquitin system is a network of proteins dedicated to the ubiquitylation of cellular targets and the subsequent control of numerous cellular functions. The deregulation of components of this elaborate network leads to human pathogenesis, including the development of many types of tumour. Alterations in the ubiquitin system that occur during the initiation and progression of cancer are now being uncovered, and this knowledge is starting to be exploited for both molecular diagnostics and the development of novel strategies to combat cancer.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Hershko, A. & Ciechanover, A. The ubiquitin system. Annu. Rev. Biochem. 67, 425–479 (1998).
Bennett, E. J. & Harper, J. W. DNA damage: ubiquitin marks the spot. Nature Struct. Mol. Biol. 15, 20–22 (2008).
Katzmann, D. J., Odorizzi, G. & Emr, S. D. Receptor downregulation and multivesicular-body sorting. Nature Rev. Mol. Cell Biol. 3, 893–905 (2002).
Haglund, K. & Dikic, I. Ubiquitylation and cell signaling. EMBO J. 24, 3353–3359 (2005).
Mukhopadhyay, D. & Riezman, H. Proteasome-independent functions of ubiquitin in endocytosis and signaling. Science 315, 201–205 (2007).
Pickart, C. M. Mechanisms underlying ubiquitination. Annu. Rev. Biochem. 70, 503–533 (2001).
Ikeda, F. & Dikic, I. Atypical ubiquitin chains: new molecular signals. EMBO Rep. 9, 536–542 (2008).
Hoeller, D., Hecker, C. M. & Dikic, I. Ubiquitin and ubiquitin-like proteins in cancer pathogenesis. Nature Rev. Cancer 6, 776–788 (2006).
Bernassola, F., Karin, M., Ciechanover, A. & Melino, G. The HECT family of E3 ubiquitin ligases: multiple players in cancer development. Cancer Cell 14, 10–21 (2008).
Nakayama, K. I. & Nakayama, K. Ubiquitin ligases: cell-cycle control and cancer. Nature Rev. Cancer 6, 369–381 (2006).
Bond, G. L., Hu, W. & Levine, A. J. MDM2 is a central node in the p53 pathway: 12 years and counting. Curr. Cancer Drug Targets 5, 3–8 (2005).
Vousden, K. H. & Prives, C. p53 and prognosis: new insights and further complexity. Cell 120, 7–10 (2005).
Cardozo, T. & Pagano, M. Wrenches in the works: drug discovery targeting the SCF ubiquitin ligase and APC/C complexes. BMC Biochem. 8 (suppl. 1), S9 (2007).
Vassilev, L. T. MDM2 inhibitors for cancer therapy. Trends Mol. Med. 13, 23–31 (2007).
Guedat, P. & Colland, F. Patented small molecule inhibitors in the ubiquitin proteasome system. BMC Biochem. 8 (suppl. 1), S14 (2007).
Hjerpe, R. & Rodriguez, M. S. Alternative UPS drug targets upstream the 26S proteasome. Int. J. Biochem. Cell Biol. 40, 1126–1140 (2008).
Yang, Y. et al. Small molecule inhibitors of HDM2 ubiquitin ligase activity stabilize and activate p53 in cells. Cancer Cell 7, 547–559 (2005). This is the first report about the development of inhibitors that target the active site of a RING-type E3. The identified compound, HLI98, shows specificity for MDM2 and allows the activation of p53-dependent transcription in cells.
VanderBorght, A. et al. Effect of an hdm-2 antagonist peptide inhibitor on cell cycle progression in p53-deficient H1299 human lung carcinoma cells. Oncogene 25, 6672–6677 (2006).
Brooks, C. L. & Gu, W. p53 ubiquitination: Mdm2 and beyond. Mol. Cell 21, 307–315 (2006).
Issaeva, N. et al. Small molecule RITA binds to p53, blocks p53–HDM-2 interaction and activates p53 function in tumors. Nature Med. 10, 1321–1328 (2004). The authors identify RITA as a potent inhibitor of p53 degradation. RITA induced p53-dependent apoptosis in various tumour cells, and showed significant antitumour effects in vivo.
Frescas, D. & Pagano, M. Deregulated proteolysis by the F-box proteins SKP2 and β-TrCP: tipping the scales of cancer. Nature Rev. Cancer 8, 438–449 (2008).
Hanahan, D. & Folkman, J. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell 86, 353–364 (1996).
Kaelin, W. G. Jr . The von Hippel–Lindau tumor suppressor protein and clear cell renal carcinoma. Clin. Cancer Res. 13, 680S–684S (2007).
Srinivasula, S. M. & Ashwell, J. D. IAPs: what's in a name? Mol. Cell 30, 123–135 (2008).
Vaux, D. L. & Silke, J. IAPs, RINGs and ubiquitylation. Nature Rev. Mol. Cell Biol. 6, 287–297 (2005).
Dierlamm, J. et al. The apoptosis inhibitor gene API2 and a novel 18q gene, MLT, are recurrently rearranged in the t(11;18)(q21;q21) associated with mucosa-associated lymphoid tissue lymphomas. Blood 93, 3601–3609 (1999).
Noels, H. et al. A novel TRAF6 binding site in MALT1 defines distinct mechanisms of NF-κB activation by API2–MALT1 fusions. J. Biol. Chem. 282, 10180–10189 (2007).
Wright, C. W. & Duckett, C. S. Reawakening the cellular death program in neoplasia through the therapeutic blockade of IAP function. J. Clin. Invest. 115, 2673–2678 (2005).
Varfolomeev, E. et al. c-IAP1 and c-IAP2 are critical mediators of TNFα-induced NF-κB activation. J. Biol. Chem. 283, 24295–24299 (2008).
Vince, J. E. et al. IAP antagonists target cIAP1 to induce TNFα-dependent apoptosis. Cell 131, 682–693 (2007). References 29 and 30 unravel the molecular mechanism of how SMAC mimetics kill cancer cells. Unexpectedly, this involves the induction of autoubiquitylation and degradation of IAPs leading to TNF-α-mediated cell death.
Cummings, J. et al. Method validation and preliminary qualification of pharmacodynamic biomarkers employed to evaluate the clinical efficacy of an antisense compound (AEG35156) targeted to the X-linked inhibitor of apoptosis protein XIAP. Br. J. Cancer 95, 42–48 (2006).
Nijman, S. M. et al. A genomic and functional inventory of deubiquitinating enzymes. Cell 123, 773–786 (2005).
Nijman, S. M. et al. The deubiquitinating enzyme USP1 regulates the Fanconi anemia pathway. Mol. Cell 17, 331–339 (2005).
Popov, N., Herold, S., Llamazares, M., Schulein, C. & Eilers, M. Fbw7 and Usp28 regulate Myc protein stability in response to DNA damage. Cell Cycle 6, 2327–2331 (2007).
Popov, N. et al. The ubiquitin-specific protease USP28 is required for MYC stability. Nature Cell Biol. 9, 765–774 (2007).
Stegmeier, F. et al. Anaphase initiation is regulated by antagonistic ubiquitination and deubiquitination activities. Nature 446, 876–881 (2007).
Wertz, I. E. et al. De-ubiquitination and ubiquitin ligase domains of A20 downregulate NF-κB signalling. Nature 430, 694–699 (2004). This paper describes the molecular mechanism of ubiquitin-chain editing by A20, a single molecule containing E3 ligase and deubiquitylating activities.
Sun, S. C. Deubiquitylation and regulation of the immune response. Nature Rev. Immunol. 8, 501–511 (2008).
Massoumi, R., Chmielarska, K., Hennecke, K., Pfeifer, A. & Fassler, R. Cyld inhibits tumor cell proliferation by blocking Bcl-3-dependent NF-κB signaling. Cell 125, 665–677 (2006).
Liu, Y. et al. Discovery of inhibitors that elucidate the role of UCH-L1 activity in the H1299 lung cancer cell line. Chem. Biol. 10, 837–846 (2003).
Love, K. R., Catic, A., Schlieker, C. & Ploegh, H. L. Mechanisms, biology and inhibitors of deubiquitinating enzymes. Nature Chem. Biol. 3, 697–705 (2007).
Rubin, D. M. & Finley, D. The proteasome: a protein-degrading organelle? Curr. Biol. 5, 854–858 (1995).
Dahlmann, B. Role of proteasomes in disease. BMC Biochem. 8 (suppl. 1), S3 (2007).
Karin, M. Nuclear factor-κB in cancer development and progression. Nature 441, 431–436 (2006).
Adams, J. The development of proteasome inhibitors as anticancer drugs. Cancer Cell 5, 417–421 (2004).
Ling, Y. H. et al. Mechanisms of proteasome inhibitor PS-341-induced G2–M-phase arrest and apoptosis in human non-small cell lung cancer cell lines. Clin. Cancer Res. 9, 1145–1154 (2003).
Meister, S. et al. Extensive immunoglobulin production sensitizes myeloma cells for proteasome inhibition. Cancer Res. 67, 1783–1792 (2007).
Gu, H., Chen, X., Gao, G. & Dong, H. Caspase-2 functions upstream of mitochondria in endoplasmic reticulum stress-induced apoptosis by bortezomib in human myeloma cells. Mol. Cancer Ther. 7, 2298–2307 (2008).
Tobinai, K. Proteasome inhibitor, bortezomib, for myeloma and lymphoma. Int. J. Clin. Oncol. 12, 318–326 (2007).
Dorsey, B. D. et al. Discovery of a potent, selective, and orally active proteasome inhibitor for the treatment of cancer. J. Med. Chem. 51, 1068–1072 (2008).
Piva, R. et al. CEP-18770: a novel, orally active proteasome inhibitor with a tumor-selective pharmacologic profile competitive with bortezomib. Blood 111, 2765–2775 (2008).
Nalepa, G., Rolfe, M. & Harper, J. W. Drug discovery in the ubiquitin–proteasome system. Nature Rev. Drug Discov. 5, 596–613 (2006).
Miller, C. P. et al. NPI-0052, a novel proteasome inhibitor, induces caspase-8 and ROS-dependent apoptosis alone and in combination with HDAC inhibitors in leukemia cells. Blood 110, 267–277 (2007).
Chauhan, D. et al. Combination of proteasome inhibitors bortezomib and NPI-0052 trigger in vivo synergistic cytotoxicity in multiple myeloma. Blood 111, 1654–1664 (2008).
Richardson, P. G. et al. Bortezomib in the front-line treatment of multiple myeloma. Expert Rev. Anticancer Ther. 8, 1053–1072 (2008).
Nickeleit, I. et al. Argyrin A reveals a critical role for the tumor suppressor protein p27kip1 in mediating antitumor activities in response to proteasome inhibition. Cancer Cell 14, 23–35 (2008).
Hashizume, R. et al. The RING heterodimer BRCA1–BARD1 is a ubiquitin ligase inactivated by a breast cancer-derived mutation. J. Biol. Chem. 276, 14537–14540 (2001).
Brooks, C. L., Li, M. & Gu, W. Monoubiquitination: the signal for p53 nuclear export? Cell Cycle 3, 436–438 (2004).
Nie, L., Sasaki, M. & Maki, C. G. Regulation of p53 nuclear export through sequential changes in conformation and ubiquitination. J. Biol. Chem. 282, 14616–14625 (2007).
Carter, S., Bischof, O., Dejean, A. & Vousden, K. H. C-terminal modifications regulate MDM2 dissociation and nuclear export of p53. Nature Cell Biol. 9, 428–435 (2007).
Moll, U. M., Wolff, S., Speidel, D. & Deppert, W. Transcription-independent pro-apoptotic functions of p53. Curr. Opin. Cell Biol. 17, 631–636 (2005).
Trotman, L. C. et al. Ubiquitination regulates PTEN nuclear import and tumor suppression. Cell 128, 141–156 (2007).
Wang, X. et al. NEDD4-1 is a proto-oncogenic ubiquitin ligase for PTEN. Cell 128, 129–139 (2007).
Cully, M., You, H., Levine, A. J. & Mak, T. W. Beyond PTEN mutations: the PI3K pathway as an integrator of multiple inputs during tumorigenesis. Nature Rev. Cancer 6, 184–192 (2006).
Gimm, O. et al. Differential nuclear and cytoplasmic expression of PTEN in normal thyroid tissue, and benign and malignant epithelial thyroid tumors. Am. J. Pathol. 156, 1693–1700 (2000).
Fouladkou, F. et al. The ubiquitin ligase Nedd4-1 is dispensable for the regulation of PTEN stability and localization. Proc. Natl Acad. Sci. USA 105, 8585–8590 (2008).
Song, M. S. et al. The deubiquitinylation and localization of PTEN are regulated by a HAUSP–PML network. Nature 455, 813–817 (2008).
Hurley, J. H., Lee, S. & Prag, G. Ubiquitin-binding domains. Biochem. J. 399, 361–372 (2006).
Bienko, M. et al. Ubiquitin-binding domains in Y-family polymerases regulate translesion synthesis. Science 310, 1821–1824 (2005).
Chen, Z. J. Ubiquitin signalling in the NF-κB pathway. Nature Cell Biol. 7, 758–765 (2005).
Verma, R. et al. Ubistatins inhibit proteasome-dependent degradation by binding the ubiquitin chain. Science 306, 117–120 (2004). Ubistatins are the first example of small molecules interfering with the recognition of specific ubiquitin chains by ubiquitin receptors.
Husnjak, K. et al. Proteasome subunit Rpn13 is a novel ubiquitin receptor. Nature 453, 481–488 (2008). This paper shows that Rpn13 is a second proteasomal ubiquitin receptor that links the recognition of ubiquitylated proteins with the disassembly of ubiquitin chains by Uch37.
Tatham, M. H. et al. Unique binding interactions among Ubc9, SUMO and RanBP2 reveal a mechanism for SUMO paralog selection. Nature Struct. Mol. Biol. 12, 67–74 (2005).
Tatham, M. H. et al. RNF4 is a poly-SUMO-specific E3 ubiquitin ligase required for arsenic-induced PML degradation. Nature Cell Biol. 10, 538–546 (2008).
Lallemand-Breitenbach, V. et al. Arsenic degrades PML or PML-RARα through a SUMO-triggered RNF4/ubiquitin-mediated pathway. Nature Cell Biol. 10, 547–555 (2008). References 74 and 75 describe the SUMO-dependent ubiquitylation of a protein and explains how arsenic-induced SUMOylation leads to degradation of the PML–RAR-α oncogene.
Acknowledgements
We thank A. Ciechanover, R. Deshaies, M. Pagano, K. Rajalingam, D. Vucic and members of the Dikic laboratory for critical reading of the manuscript. We apologize to those investigators whose contributions are not described here because of space limitations. D.H. is supported by a European Molecular Biology Organization (EMBO) long-term fellowship. I.D. acknowledges support from the German Research Foundation (DFG) and the Cluster of Excellence 'Macromolecular Complexes' (Goethe University Frankfurt).
Author information
Authors and Affiliations
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Additional information
Reprints and permissions information is available at http://www.nature.com/reprints.
Correspondence should be addressed to I.D (dikic@biochem2.uni-frankfurt.de).
Rights and permissions
About this article
Cite this article
Hoeller, D., Dikic, I. Targeting the ubiquitin system in cancer therapy. Nature 458, 438–444 (2009). https://doi.org/10.1038/nature07960
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature07960
This article is cited by
-
TRIM21/USP15 balances ACSL4 stability and the imatinib resistance of gastrointestinal stromal tumors
British Journal of Cancer (2024)
-
PSMD2 contributes to the progression of esophageal squamous cell carcinoma by repressing autophagy
Cell & Bioscience (2023)
-
USP51 promotes non-small cell lung carcinoma cell stemness by deubiquitinating TWIST1
Journal of Translational Medicine (2023)
-
PHF5A facilitates the development and progression of gastric cancer through SKP2-mediated stabilization of FOS
Journal of Translational Medicine (2023)
-
GSG2 facilitates the progression of human breast cancer through MDM2-mediated ubiquitination of E2F1
Journal of Translational Medicine (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.