Abstract
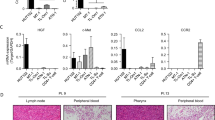
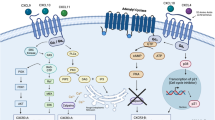
T-cell acute lymphoblastic leukaemia (T-ALL) is a blood malignancy afflicting mainly children and adolescents1. T-ALL patients present at diagnosis with increased white cell counts and hepatosplenomegaly, and are at an increased risk of central nervous system (CNS) relapse2,3. For that reason, T-ALL patients usually receive cranial irradiation in addition to intensified intrathecal chemotherapy. The marked increase in survival is thought to be worth the considerable side-effects associated with this therapy. Such complications include secondary tumours, neurocognitive deficits, endocrine disorders and growth impairment3. Little is known about the mechanism of leukaemic cell infiltration of the CNS, despite its clinical importance4. Here we show, using T-ALL animal modelling and gene-expression profiling, that the chemokine receptor CCR7 (ref. 5) is the essential adhesion signal required for the targeting of leukaemic T-cells into the CNS. Ccr7 gene expression is controlled by the activity of the T-ALL oncogene Notch1 and is expressed in human tumours carrying Notch1-activating mutations. Silencing of either CCR7 or its chemokine ligand CCL19 (ref. 6) in an animal model of T-ALL specifically inhibits CNS infiltration. Furthermore, murine CNS-targeting by human T-ALL cells depends on their ability to express CCR7. These studies identify a single chemokine–receptor interaction as a CNS ‘entry’ signal, and open the way for future pharmacological targeting. Targeted inhibition of CNS involvement in T-ALL could potentially decrease the intensity of CNS-targeted therapy, thus reducing its associated short- and long-term complications.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Grabher, C., von Boehmer, H. & Look, A. T. Notch 1 activation in the molecular pathogenesis of T-cell acute lymphoblastic leukaemia. Nature Rev. Cancer 6, 347–359 (2006)
Aifantis, I., Raetz, E. & Buonamici, S. Molecular pathogenesis of T-cell leukaemia and lymphoma. Nature Rev. Immunol. 8, 380–390 (2008)
Pui, C. H. & Howard, S. C. Current management and challenges of malignant disease in the CNS in paediatric leukaemia. Lancet Oncol. 9, 257–268 (2008)
Pui, C. H. & Evans, W. E. Treatment of acute lymphoblastic leukemia. N. Engl. J. Med. 354, 166–178 (2006)
Cyster, J. G. Chemokines, sphingosine-1-phosphate, and cell migration in secondary lymphoid organs. Annu. Rev. Immunol. 23, 127–159 (2005)
Forster, R., Davalos-Misslitz, A. C. & Rot, A. CCR7 and its ligands: balancing immunity and tolerance. Nature Rev. Immunol. 8, 362–371 (2008)
Weng, A. P. et al. Activating mutations of NOTCH1 in human T cell acute lymphoblastic leukemia. Science 306, 269–271 (2004)
Thompson, B. J. et al. The SCFFBW7 ubiquitin ligase complex as a tumor suppressor in T cell leukemia. J. Exp. Med. 204, 1825–1835 (2007)
Palomero, T. et al. Mutational loss of PTEN induces resistance to NOTCH1 inhibition in T-cell leukemia. Nature Med. 13, 1203–1210 (2007)
O’Neil, J. et al. FBW7 mutations in leukemic cells mediate NOTCH pathway activation and resistance to γ-secretase inhibitors. J. Exp. Med. 204, 1813–1824 (2007)
Vilimas, T. et al. Targeting the NF-κB signaling pathway in Notch1-induced T-cell leukemia. Nature Med. 13, 70–77 (2007)
Klinakis, A. et al. Igf1r as a therapeutic target in a mouse model of basal-like breast cancer. Proc. Natl Acad. Sci. USA 106, 2359–2364 (2009)
Charo, I. F. & Ransohoff, R. M. The many roles of chemokines and chemokine receptors in inflammation. N. Engl. J. Med. 354, 610–621 (2006)
Cardona, A. E. L. i. M., Liu, L., Savarin, C. & Ransohoff, R. M. Chemokines in and out of the central nervous system: much more than chemotaxis and inflammation. J. Leukoc. Biol. 84, 587–594 (2008)
Muller, A. et al. Involvement of chemokine receptors in breast cancer metastasis. Nature 410, 50–56 (2001)
Shields, J. D. et al. Autologous chemotaxis as a mechanism of tumor cell homing to lymphatics via interstitial flow and autocrine CCR7 signaling. Cancer Cell 11, 526–538 (2007)
Forster, R. et al. CCR7 coordinates the primary immune response by establishing functional microenvironments in secondary lymphoid organs. Cell 99, 23–33 (1999)
Mempel, T. R., Scimone, M. L., Mora, J. R. & von Andrian, U. H. In vivo imaging of leukocyte trafficking in blood vessels and tissues. Curr. Opin. Immunol. 16, 406–417 (2004)
Kivisakk, P. et al. Human cerebrospinal fluid central memory CD4+ T cells: evidence for trafficking through choroid plexus and meninges via P-selectin. Proc. Natl Acad. Sci. USA 100, 8389–8394 (2003)
Giunti, D. et al. Phenotypic and functional analysis of T cells homing into the CSF of subjects with inflammatory diseases of the CNS. J. Leukoc. Biol. 73, 584–590 (2003)
Kivisakk, P. et al. Expression of CCR7 in multiple sclerosis: implications for CNS immunity. Ann. Neurol. 55, 627–638 (2004)
Gunn, M. D. et al. Mice lacking expression of secondary lymphoid organ chemokine have defects in lymphocyte homing and dendritic cell localization. J. Exp. Med. 189, 451–460 (1999)
Ransohoff, R. M. Natalizumab for multiple sclerosis. N. Engl. J. Med. 356, 2622–2629 (2007)
Tseng, J. C. et al. Tumor-specific in vivo transfection with HSV-1 thymidine kinase gene using a Sindbis viral vector as a basis for prodrug ganciclovir activation and PET. J. Nucl. Med. 47, 1136–1143 (2006)
Shakhar, G. et al. Stable T cell-dendritic cell interactions precede the development of both tolerance and immunity in vivo . Nature Immunol. 6, 707–714 (2005)
Mempel, T. R., Henrickson, S. E. & Von Andrian, U. H. T-cell priming by dendritic cells in lymph nodes occurs in three distinct phases. Nature 427, 154–159 (2004)
Sicinska, E. et al. Requirement for cyclin D3 in lymphocyte development and T cell leukemias. Cancer Cell 4, 451–461 (2003)
Ory, D. S., Neugeboren, B. A. & Mulligan, R. C. A stable human-derived packaging cell line for production of high titer retrovirus/vesicular stomatitis virus G pseudotypes. Proc. Natl Acad. Sci. USA 93, 11400–11406 (1996)
Scimone, M. L., Aifantis, I., Apostolou, I., von Boehmer, H. & von Andrian, U. H. A multistep adhesion cascade for lymphoid progenitor cell homing to the thymus. Proc. Natl Acad. Sci. USA 103, 7006–7011 (2006)
Acknowledgements
We are grateful to G. Randolph, E. Kuan and T. Vilimas for technical help and discussions. We would like to thank P. Lopez and G. De La Cruz for cell sorting; D. Littman, W. Carroll, E. Raetz, S. Lira and S. Schwab for advice and illuminating discussions; C. Loomis and the Histology Facility for advice and troubleshooting tips. This work was supported by the National Institutes of Health (RO1CA105129, RO1CA133379, R56AI070310, P30CA016087 to I.A., RO1AI41428, RO1AI072039 to J.S.B.), the American Cancer Society (RSG0806801 to I.A.), the Dana Foundation, The Chemotherapy Foundation, the Alex’s Lemonade Stand Foundation (to I.A.), the Lauri Strauss Leukemia Foundation (to F.S.), the G&P Foundation, NYU Molecular Oncology and Immunology training grant (to S.B.), American Society of Hematology (to S.B.), Juvenile Diabetes Research Foundation (JDRFI1-2008-90 and 5-2008-236 to J.S.B.), the National Cancer Institute (1 P01 CA97403, Project 2 to A.E.) and a gift from the Berrie Foundation (to A.E.). A.K. was supported by a Fellowship from the Jane Coffin Childs Memorial Fund for Medical Research.
Author Contributions S.B., B.G.M. and I.A. designed experiments, performed experiments and wrote the manuscript. M.L.D., M.Li and M.G.R. performed the in vivo two-photon microscopy experiments and analysis. D.M. and J.-C.T. performed the bioluminescent imaging experiments. A.K. and A.E. generated the EF1-Notch1-IC mice. J.Z. performed the microarray data mining. Y.L., E.N. and D.Z. performed in the CNS pathology analysis. M.Lipp and J.S.B. assisted with experimental design, provided reagents and advice. T.T., L.R., S.C. and E.G. performed experiments.
Author information
Authors and Affiliations
Corresponding author
Supplementary information
Supplementary Information
This file contains Supplementary Figures 1-12 with Legends and Supplementary Table 1. (PDF 9868 kb)
Supplementary Movie 1
This movie is a time-lapse movie acquired by using a 2-photon microscope of WTNI-IC cells in the lymph nodes. Leukemic cells are in green (EGFP), blood vessels are in red (Quantum Dots), in blue is the capsule of the lymph node (second harmonic). (MOV 2319 kb)
Supplementary Movie 2
This file is a time-lapse movie acquired by using a 2-photon microscope of CCR7-KON1-IC cells in the lymph nodes. Leukemic cells are in green (EGFP), blood vessels are in red (Quantum Dots), in blue is the capsule of the lymph node (second harmonic). (MOV 3150 kb)
Rights and permissions
About this article
Cite this article
Buonamici, S., Trimarchi, T., Ruocco, M. et al. CCR7 signalling as an essential regulator of CNS infiltration in T-cell leukaemia. Nature 459, 1000–1004 (2009). https://doi.org/10.1038/nature08020
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nature08020
This article is cited by
-
Lymphatic endothelial-like cells promote glioblastoma stem cell growth through cytokine-driven cholesterol metabolism
Nature Cancer (2024)
-
Interactome based identification and validation of prefoldin 5-α for prognosing CNS leukemia in B-ALL patients
Scientific Reports (2022)
-
Application of Metabolomics in Childhood Leukemia Diagnostics
Archivum Immunologiae et Therapiae Experimentalis (2022)
-
Central Nervous System Involvement in Adults with Acute Leukemia: Diagnosis, Prevention, and Management
Current Oncology Reports (2022)
-
Targeting chemokines for acute lymphoblastic leukemia therapy
Journal of Hematology & Oncology (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.