Abstract
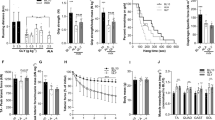
Duchenne muscular dystrophy (DMD) is a severe and progressive muscle wasting disorder caused by mutations in the dystrophin gene that result in the absence of the membrane-stabilizing protein dystrophin1,2,3. Dystrophin-deficient muscle fibres are fragile and susceptible to an influx of Ca2+, which activates inflammatory and muscle degenerative pathways4,5,6. At present there is no cure for DMD, and existing therapies are ineffective. Here we show that increasing the expression of intramuscular heat shock protein 72 (Hsp72) preserves muscle strength and ameliorates the dystrophic pathology in two mouse models of muscular dystrophy. Treatment with BGP-15 (a pharmacological inducer of Hsp72 currently in clinical trials for diabetes) improved muscle architecture, strength and contractile function in severely affected diaphragm muscles in mdx dystrophic mice. In dko mice, a phenocopy of DMD that results in severe spinal curvature (kyphosis), muscle weakness and premature death7,8, BGP-15 decreased kyphosis, improved the dystrophic pathophysiology in limb and diaphragm muscles and extended lifespan. We found that the sarcoplasmic/endoplasmic reticulum Ca2+-ATPase (SERCA, the main protein responsible for the removal of intracellular Ca2+) is dysfunctional in severely affected muscles of mdx and dko mice, and that Hsp72 interacts with SERCA to preserve its function under conditions of stress, ultimately contributing to the decreased muscle degeneration seen with Hsp72 upregulation. Treatment with BGP-15 similarly increased SERCA activity in dystrophic skeletal muscles. Our results provide evidence that increasing the expression of Hsp72 in muscle (through the administration of BGP-15) has significant therapeutic potential for DMD and related conditions, either as a self-contained therapy or as an adjuvant with other potential treatments, including gene, cell and pharmacological therapies.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Emery, A. E. The muscular dystrophies. Lancet 359, 687–695 (2002)
Ervasti, J. M. & Campbell, K. P. Membrane organization of the dystrophin–glycoprotein complex. Cell 66, 1121–1131 (1991)
Koenig, M., Monaco, A. P. & Kunkel, L. M. The complete sequence of dystrophin predicts a rod-shaped cytoskeletal protein. Cell 53, 219–228 (1988)
Blake, D. J., Weir, A., Newey, S. E. & Davies, K. E. Function and genetics of dystrophin and dystrophin-related proteins in muscle. Physiol. Rev. 82, 291–329 (2002)
Straub, V., Rafael, J. A., Chamberlain, J. S. & Campbell, K. P. Animal models for muscular dystrophy show different patterns of sarcolemmal disruption. J. Cell Biol. 139, 375–385 (1997)
Turner, P. R., Westwood, T., Regen, C. M. & Steinhardt, R. A. Increased protein degradation results from elevated free calcium levels found in muscle from mdx mice. Nature 335, 735–738 (1988)
Grady, R. M. et al. Skeletal and cardiac myopathies in mice lacking utrophin and dystrophin: a model for Duchenne muscular dystrophy. Cell 90, 729–738 (1997)
Deconinck, A. E. et al. Utrophin-dystrophin-deficient mice as a model for Duchenne muscular dystrophy. Cell 90, 717–727 (1997)
Gervasio, O. L., Whitehead, N. P., Yeung, E. W., Phillips, W. D. & Allen, D. G. TRPC1 binds to caveolin-3 and is regulated by Src kinase—role in Duchenne muscular dystrophy. J. Cell Sci. 121, 2246–2255 (2008)
Fong, P. Y., Turner, P. R., Denetclaw, W. F. & Steinhardt, R. A. Increased activity of calcium leak channels in myotubes of Duchenne human and mdx mouse origin. Science 250, 673–676 (1990)
Bellinger, A. M. et al. Hypernitrosylated ryanodine receptor calcium release channels are leaky in dystrophic muscle. Nature Med. 15, 325–330 (2009)
Nicolas-Metral, V., Raddatz, E., Kucera, P. & Ruegg, U. T. Mdx myotubes have normal excitability but show reduced contraction–relaxation dynamics. J. Muscle Res. Cell Motil. 22, 69–75 (2001)
Tutdibi, O., Brinkmeier, H., Rudel, R. & Fohr, K. J. Increased calcium entry into dystrophin-deficient muscle fibres of MDX and ADR-MDX mice is reduced by ion channel blockers. J. Physiol. (Lond.) 515, 859–868 (1999)
Goonasekera, S. A. et al. Mitigation of muscular dystrophy in mice by SERCA overexpression in skeletal muscle. J. Clin. Invest. 121, 1044–1052 (2011)
Morine, K. J., Sleeper, M. M., Barton, E. R. & Sweeney, H. L. Overexpression of SERCA1a in the mdx diaphragm reduces susceptibility to contraction-induced damage. Hum. Gene Ther. 12, 1735–1739 (2010)
Porter, J. D. et al. A chronic inflammatory response dominates the skeletal muscle molecular signature in dystrophin-deficient mdx mice. Hum. Mol. Genet. 11, 263–272 (2002)
Acharyya, S. et al. Interplay of IKK/NF-κB signaling in macrophages and myofibres promotes muscle degeneration in Duchenne muscular dystrophy. J. Clin. Invest. 117, 889–901 (2007)
Kolodziejczyk, S. M. et al. Activation of JNK1 contributes to dystrophic muscle pathogenesis. Curr. Biol. 11, 1278–1282 (2001)
Monici, M. C., Aguennouz, M., Mazzeo, A., Messina, C. & Vita, G. Activation of nuclear factor-κB in inflammatory myopathies and Duchenne muscular dystrophy. Neurology 60, 993–997 (2003)
Senf, S. M., Dodd, S. L., McClung, J. M. & Judge, A. R. Hsp70 overexpression inhibits NF-κB and Foxo3a transcriptional activities and prevents skeletal muscle atrophy. FASEB J. 22, 3836–3845 (2008)
Park, H. S., Lee, J. S., Huh, S. H., Seo, J. S. & Choi, E. J. Hsp72 functions as a natural inhibitory protein of c-Jun N-terminal kinase. EMBO J. 20, 446–456 (2001)
Chung, J. et al. HSP72 protects against obesity-induced insulin resistance. Proc. Natl Acad. Sci. USA 105, 1739–1744 (2008)
Tupling, A. R. et al. HSP70 binds to the fast-twitch skeletal muscle sarco(endo)plasmic reticulum Ca2+-ATPase (SERCA1a) and prevents thermal inactivation. J. Biol. Chem. 279, 52382–52389 (2004)
Bornman, L., Polla, B. S., Lotz, B. P. & Gericke, G. S. Expression of heat-shock/stress proteins in Duchenne muscular dystrophy. Muscle Nerve 18, 23–31 (1995)
Lou, J. S., Weiss, M. D. & Carter, G. T. Assessment and management of fatigue in neuromuscular disease. Am. J. Hosp. Palliat. Care 27, 145–157 (2010)
Finsterer, J. Cardiopulmonary support in Duchenne muscular dystrophy. Lung 184, 205–215 (2006)
Stedman, H. H. et al. The mdx mouse diaphragm reproduces the degenerative changes of Duchenne muscular dystrophy. Nature 352, 536–539 (1991)
Briguet, A., Courdier-Fruh, I., Foster, M., Meier, T. & Magyar, J. P. Histological parameters for the quantitative assessment of muscular dystrophy in the mdx-mouse. Neuromuscul. Disord. 14, 675–682 (2004)
Literati-Nagy, B. et al. Improvement of insulin sensitivity by a novel drug, BGP-15, in insulin-resistant patients: a proof of concept randomized double-blind clinical trial. Horm. Metab. Res. 41, 374–380 (2009)
Morimoto, R. I. Cells in stress: transcriptional activation of heat shock genes. Science 259, 1409–1410 (1993)
Marber, M. S. et al. Overexpression of the rat inducible 70-kD heat stress protein in a transgenic mouse increases the resistance of the heart to ischemic injury. J. Clin. Invest. 95, 1446–1456 (1995)
Taleb, M. et al. Hsp70 inhibits aminoglycoside-induced hair cell death and is necessary for the protective effect of heat shock. J. Assoc. Res. Otolaryngol. 9, 277–289 (2008)
Literati-Nagy, B. et al. Beneficial effect of the insulin sensitizer (HSP inducer) BGP-15 on olanzapine-induced metabolic disorders. Brain Res. Bull. 20, 340–344 (2010)
Saegusa, Y. & Tabata, H. Usefulness of infrared thermometry in determining body temperature in mice. J. Vet. Med. Sci. 65, 1365–1367 (2003)
Gregorevic, P., Plant, D. R., Leeding, K. S., Bach, L. A. & Lynch, G. S. Improved contractile function of the mdx dystrophic mouse diaphragm muscle after insulin-like growth factor-I administration. Am. J. Pathol. 161, 2263–2272 (2002)
Schertzer, J. D., Ryall, J. G. & Lynch, G. S. Systemic administration of IGF-I enhances oxidative status and reduces contraction-induced injury in skeletal muscles of mdx dystrophic mice. Am. J. Physiol. 291, 499–505 (2006)
Schertzer, J. D., Gehrig, S. M., Ryall, J. G. & Lynch, G. S. Modulation of insulin-like growth factor (IGF)-I and IGF-binding protein interactions enhances skeletal muscle regeneration and ameliorates the dystrophic pathology in mdx mice. Am. J. Pathol. 171, 1180–1188 (2007)
Gehrig, S. M., Koopman, R., Naim, T., Tjoakarfa, C. & Lynch, G. S. Making fast-twitch dystrophic muscles bigger protects them from contraction injury and attenuates the dystrophic pathology. Am. J. Pathol. 176, 29–33 (2010)
Briguet, A., Courdier-Fruh, I., Foster, M., Meier, T. & Magyar, J. P. Histological parameters for the quantitative assessment of muscular dystrophy in the mdx-mouse. Neuromuscul. Disord. 14, 675–682 (2004)
Bradford, M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72, 248–254 (1976)
Schertzer, J. D. et al. β2-Agonist administration increases sarcoplasmic reticulum Ca2+-ATPase activity in aged rat skeletal muscle. Am. J. Physiol. 288, 526–533 (2005)
Plant, D. R. & Lynch, G. S. Depolarization-induced contraction and SR function in mechanically skinned muscle fibres from dystrophic mdx mice. Am. J. Physiol. 285, C522–C528 (2003)
Murphy, K. T. et al. Antibody-directed myostatin inhibition in 21-mo-old mice reveals novel roles for myostatin signaling in skeletal muscle structure and function. FASEB J. 24, 4433–4442 (2010)
Green, M. C. A rapid method for clearing and staining specimens for the demonstration of bone. Ohio J. Sci. 52, 31–33 (1952)
Acknowledgements
We thank R. Koopman, J. G. Ryall and G. I. Lancaster for comments; J. Trieu, B. G. Gleeson, T. Naim and A. Chee for technical support; and C. Angelini and the Neuromuscular bank of tissues and DNA samples – Telethon Network of Genetic Biobanks for the provision of human muscle specimens. We thank N-Gene R&D Inc. USA for providing the BGP-15 compound. This study was supported in part by research grants from the National Health and Medical Research Council (NHMRC; project numbers 1009114 to G.S.L. and 472650 and 1004441 to M.A.F.), Association Française contre les Myopathies (France, to G.S.L.) and the Muscular Dystrophy Association (USA, to G.S.L.). M.A.F. is a Senior Principal Research Fellow of the NHMRC. A.P.R. was supported by a NHMRC Biomedical career Development Award. S.L. was supported by a postdoctoral fellowship from the Swiss National Science Foundation. S.M.G. was supported by a National Heart Foundation Postgraduate Scholarship (Australia). D.C.H. was supported by a National Heart Foundation Post-Doctoral Fellowship.
Author information
Authors and Affiliations
Contributions
S.M.G., J.D.S., C.v.d.P., M.A.F. and G.S.L. conceived and designed the experiments. S.M.G., T.A.S., C.v.d.P., D.C.H. and J.E.C. performed the experiments. M.A.F. and K.E.D. facilitated experiments through the provision of mice and experimental compounds. A.P.R. and S.L. performed experiments on muscle samples from patients with DMD and from controls. S.M.G., D.C.H., M.A.F., C.v.d.P. and G.S.L. analysed the data. S.M.G. and G.S.L. wrote the manuscript. All authors checked for scientific content and contributed to the final drafting of the manuscript.
Corresponding author
Ethics declarations
Competing interests
M.A.F. is a scientific consultant for N-Gene R&D Inc. USA, who supplied the BGP-15 compound. He has a position on a patent held by N-Gene R&D Inc. D.C.H., G.S.L. and S.M.G. have positions on a patent held by N-Gene R&D Inc. The remaining authors declare no competing interests.
Supplementary information
Supplementary Information
This file contains Supplementary Figures 1-8 and Supplementary Table 1. (PDF 1465 kb)
Rights and permissions
About this article
Cite this article
Gehrig, S., van der Poel, C., Sayer, T. et al. Hsp72 preserves muscle function and slows progression of severe muscular dystrophy. Nature 484, 394–398 (2012). https://doi.org/10.1038/nature10980
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature10980
This article is cited by
-
HALD, a human aging and longevity knowledge graph for precision gerontology and geroscience analyses
Scientific Data (2023)
-
Aging of mesenchymal stem cell: machinery, markers, and strategies of fighting
Cellular & Molecular Biology Letters (2022)
-
Neprilysins regulate muscle contraction and heart function via cleavage of SERCA-inhibitory micropeptides
Nature Communications (2022)
-
The SarcoEndoplasmic Reticulum Calcium ATPase (SERCA) pump: a potential target for intervention in aging and skeletal muscle pathologies
Skeletal Muscle (2021)
-
Identification of biomarkers for physical frailty and sarcopenia through a new multi-marker approach: results from the BIOSPHERE study
GeroScience (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.