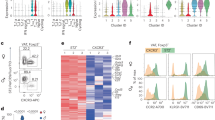
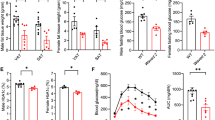
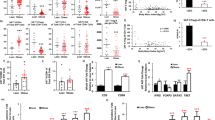
Abstract
Obesity and type-2 diabetes have increased markedly over the past few decades, in parallel. One of the major links between these two disorders is chronic, low-grade inflammation1. Prolonged nutrient excess promotes the accumulation and activation of leukocytes in visceral adipose tissue (VAT) and ultimately other tissues, leading to metabolic abnormalities such as insulin resistance, type-2 diabetes and fatty-liver disease. Although invasion of VAT by pro-inflammatory macrophages is considered to be a key event driving adipose-tissue inflammation and insulin resistance, little is known about the roles of other immune system cell types in these processes. A unique population of VAT-resident regulatory T (Treg) cells was recently implicated in control of the inflammatory state of adipose tissue and, thereby, insulin sensitivity2. Here we identify peroxisome proliferator-activated receptor (PPAR)-γ, the ‘master regulator’ of adipocyte differentiation, as a crucial molecular orchestrator of VAT Treg cell accumulation, phenotype and function. Unexpectedly, PPAR-γ expression by VAT Treg cells was necessary for complete restoration of insulin sensitivity in obese mice by the thiazolidinedione drug pioglitazone. These findings suggest a previously unknown cellular mechanism for this important class of thiazolidinedione drugs, and provide proof-of-principle that discrete populations of Treg cells with unique functions can be precisely targeted to therapeutic ends.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Osborn, O. & Olefsky, J. M. The cellular and signaling networks linking the immune system and metabolism in disease. Nature Med. 18, 363–374 (2012)
Feuerer, M. et al. Lean, but not obese, fat is enriched for a unique population of regulatory T cells that affect metabolic parameters. Nature Med. 15, 930–939 (2009)
Winer, S. et al. Normalization of obesity-associated insulin resistance through immunotherapy. Nature Med. 15, 921–929 (2009)
Ilan, Y. et al. Induction of regulatory T cells decreases adipose inflammation and alleviates insulin resistance in ob/ob mice. Proc. Natl Acad. Sci. USA 107, 9765–9770 (2010)
Tontonoz, P. & Spiegelman, B. M. Fat and beyond: the diverse biology of PPARgamma. Annu. Rev. Biochem. 77, 289–312 (2008)
Tontonoz, P. et al. PPARγ promotes monocyte/macrophage differentiation and uptake of oxidized LDL. Cell 93, 241–252 (1998)
Miyazaki, Y. et al. Effect of pioglitazone on circulating adipocytokine levels and insulin sensitivity in type 2 diabetic patients. J. Clin. Endocrinol. Metab. 89, 4312–4319 (2004)
Sugii, S. et al. PPARγ activation in adipocytes is sufficient for systemic insulin sensitization. Proc. Natl Acad. Sci. USA 106, 22504–22509 (2009)
Hevener, A. L. et al. Macrophage PPARγ is required for normal skeletal muscle and hepatic insulin sensitivity and full antidiabetic effects of thiazolidinediones. J. Clin. Invest. 117, 1658–1669 (2007)
Odegaard, J. I. et al. Macrophage-specific PPARγ controls alternative activation and improves insulin resistance. Nature 447, 1116–1120 (2007)
Hevener, A. L. et al. Muscle-specific Pparg deletion causes insulin resistance. Nature Med. 9, 1491–1497 (2003)
Ryan, K. K. et al. A role for central nervous system PPAR-γ in the regulation of energy balance. Nature Med. 17, 623–626 (2011)
Lu, M. et al. Brain PPAR-γ promotes obesity and is required for the insulin-sensitizing effect of thiazolidinediones. Nature Med. 17, 618–622 (2011)
Olefsky, J. M., Lazar, M. A. & Scherer, P. E. Antidiabetes wars: a new hope. Nature Med. 16, 972–973 (2010)
Josefowicz, S. Z., Lu, L. F. & Rudensky, A. Y. Regulatory T cells: mechanisms of differentiation and function. Annu. Rev. Immunol. 30, 531–563 (2012)
Rubtsov, Y. P. et al. Regulatory T cell-derived interleukin-10 limits inflammation at environmental interfaces. Immunity 28, 546–558 (2008)
Akiyama, T. E. et al. Conditional disruption of the peroxisome proliferator-activated receptor γ gene in mice results in lowered expression of ABCA1, ABCG1, and apoE in macrophages and reduced cholesterol efflux. Mol. Cell Biol. 22, 2607–2619 (2002)
Morita, S., Kojima, T. & Kitamura, T. Plat-E: an efficient and stable system for transient packaging of retroviruses. Gene Ther. 7, 1063–1066 (2000)
Holst, J. et al. Generation of T-cell receptor retrogenic mice. Nature Protoc. 1, 406–417 (2006)
Naviaux, R. K., Costanzi, E., Haas, M. & Verma, I. M. The pCL vector system: rapid production of helper-free, high-titer, recombinant retroviruses. J. Virol. 70, 5701–5705 (1996)
Leesnitzer, L. M. et al. Functional consequences of cysteine modification in the ligand binding sites of peroxisome proliferator activated receptors by GW9662. Biochemistry 41, 6640–6650 (2002)
Reich, M. et al. GenePattern 2.0. Nature Genet. 38, 500–501 (2006)
Heng, T. S. & Painter, M. W. The Immunological Genome Project: networks of gene expression in immune cells. Nature Immunol. 9, 1091–1094 (2008)
Acknowledgements
We thank A. Rudensky, F. Gonzalez, R. Kahn, B. Spiegelman and D. Vignali for providing materials; K. Hattori, M. Davenport, J. LaVecchio, G. Buruzala, J. Ericson, K. Leatherbee and S. Davis for technical assistance; A. Ergun, A. Morton and J. Shu for experimental help; and M. Wilson and J. Hill for discussions. Supported by grants from the National Institutes of Health (NIH; DK092541) and Ellison Foundation (Boston) to D.M. and C.B., Dana Foundation to D.M. and S.E.S.; the American Diabetes Association (RA 110BS97) to J.L., and the NIH (DK51729) to S.E.S.; as well as by core facilities of the Joslin Diabetes Center (P30DK36836). M.F. received a postdoctoral fellowship from the King Trust.
Author information
Authors and Affiliations
Contributions
All authors designed research; D.C. and A.L. performed research; D.C., S.E.S., C.B. and D.M. analysed data; D.C., C.B. and D.M. wrote the paper.
Corresponding author
Ethics declarations
Competing interests
D.M., C.B., M.F. and S.E.S. have a patent pending on fat Treg cells.
Supplementary information
Supplementary Information
This file contains Supplementary Figures1-9. (PDF 2543 kb)
Rights and permissions
About this article
Cite this article
Cipolletta, D., Feuerer, M., Li, A. et al. PPAR-γ is a major driver of the accumulation and phenotype of adipose tissue Treg cells. Nature 486, 549–553 (2012). https://doi.org/10.1038/nature11132
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature11132
This article is cited by
-
15-Lipoxygenase promotes resolution of inflammation in lymphedema by controlling Treg cell function through IFN-β
Nature Communications (2024)
-
Macrophage and T cell networks in adipose tissue
Nature Reviews Endocrinology (2024)
-
Insight into the significance of Foxp3 + tumor-infiltrating lymphocytes in squamous cell lung cancer
Clinical and Translational Oncology (2024)
-
Pparα knockout in mice increases the Th17 development by facilitating the IKKα/RORγt and IKKα/Foxp3 complexes
Communications Biology (2023)
-
The role of transcription factors in shaping regulatory T cell identity
Nature Reviews Immunology (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.