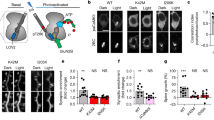
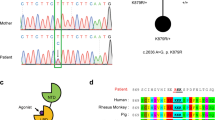
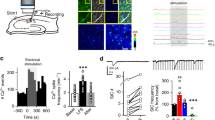
Abstract
Long-term potentiation (LTP) of synaptic transmission is thought to be an important cellular mechanism underlying memory formation. A widely accepted model posits that LTP requires the cytoplasmic carboxyl tail (C-tail) of the AMPA (α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid) receptor subunit GluA1. To find the minimum necessary requirement of the GluA1 C-tail for LTP in mouse CA1 hippocampal pyramidal neurons, we used a single-cell molecular replacement strategy to replace all endogenous AMPA receptors with transfected subunits. In contrast to the prevailing model, we found no requirement of the GluA1 C-tail for LTP. In fact, replacement with the GluA2 subunit showed normal LTP, as did an artificially expressed kainate receptor not normally found at these synapses. The only conditions under which LTP was impaired were those with markedly decreased AMPA receptor surface expression, indicating a requirement for a reserve pool of receptors. These results demonstrate the synapse’s remarkable flexibility to potentiate with a variety of glutamate receptor subtypes, requiring a fundamental change in our thinking with regard to the core molecular events underlying synaptic plasticity.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Hollmann, M. & Heinemann, S. Cloned glutamate receptors. Annu. Rev. Neurosci. 17, 31–108 (1994)
Wisden, W. & Seeburg, P. H. Mammalian ionotropic glutamate receptors. Curr. Opin. Neurobiol. 3, 291–298 (1993)
Lu, W. et al. Subunit composition of synaptic AMPA receptors revealed by a single-cell genetic approach. Neuron 62, 254–268 (2009)
Wenthold, R. J., Petralia, R. S., Blahos, J., II & Niedzielski, A. S. Evidence for multiple AMPA receptor complexes in hippocampal CA1/CA2 neurons. J. Neurosci. 16, 1982–1989 (1996)
Shi, S., Hayashi, Y., Esteban, J. A. & Malinow, R. Subunit-specific rules governing AMPA receptor trafficking to synapses in hippocampal pyramidal neurons. Cell 105, 331–343 (2001)
Boehm, J. et al. Synaptic incorporation of AMPA receptors during LTP is controlled by a PKC phosphorylation site on GluR1. Neuron 51, 213–225 (2006)
Hayashi, Y. et al. Driving AMPA receptors into synapses by LTP and CaMKII: requirement for GluR1 and PDZ domain interaction. Science 287, 2262–2267 (2000)
Zamanillo, D. et al. Importance of AMPA receptors for hippocampal synaptic plasticity but not for spatial learning. Science 284, 1805–1811 (1999)
Meng, Y., Zhang, Y. & Jia, Z. Synaptic transmission and plasticity in the absence of AMPA glutamate receptor GluR2 and GluR3. Neuron 39, 163–176 (2003)
Kessels, H. W. & Malinow, R. Synaptic AMPA receptor plasticity and behavior. Neuron 61, 340–350 (2009)
Anggono, V. & Huganir, R. L. Regulation of AMPA receptor trafficking and synaptic plasticity. Curr. Opin. Neurobiol. 22, 461–469 (2012)
Collingridge, G. L., Isaac, J. T. & Wang, Y. T. Receptor trafficking and synaptic plasticity. Nature Rev. Neurosci. 5, 952–962 (2004)
Malenka, R. C. & Bear, M. F. LTP and LTD: an embarrassment of riches. Neuron 44, 5–21 (2004)
Malenka, R. C. Synaptic plasticity and AMPA receptor trafficking. Ann. NY Acad. Sci. 1003, 1–11 (2003)
Malinow, R. & Malenka, R. C. AMPA receptor trafficking and synaptic plasticity. Annu. Rev. Neurosci. 25, 103–126 (2002)
Bredt, D. S. & Nicoll, R. A. AMPA receptor trafficking at excitatory synapses. Neuron 40, 361–379 (2003)
Shepherd, J. D. & Huganir, R. L. The cell biology of synaptic plasticity: AMPA receptor trafficking. Annu. Rev. Cell Dev. Biol. 23, 613–643 (2007)
Granger, A. J., Gray, J. A., Lu, W. & Nicoll, R. A. Genetic analysis of neuronal ionotropic glutamate receptor subunits. J. Physiol. (Lond.) 589, 4095–4101 (2011)
Lu, W., Isozaki, K., Roche, K. W. & Nicoll, R. A. Synaptic targeting of AMPA receptors is regulated by a CaMKII site in the first intracellular loop of GluA1. Proc. Natl Acad. Sci. USA 107, 22266–22271 (2010)
Andrasfalvy, B. K., Smith, M. A., Borchardt, T., Sprengel, R. & Magee, J. C. Impaired regulation of synaptic strength in hippocampal neurons from GluR1-deficient mice. J. Physiol. (Lond.) 552, 35–45 (2003)
Panicker, S., Brown, K. & Nicoll, R. A. Synaptic AMPA receptor subunit trafficking is independent of the C terminus in the GluR2-lacking mouse. Proc. Natl Acad. Sci. USA 105, 1032–1037 (2008)
Shen, L., Liang, F., Walensky, L. D. & Huganir, R. L. Regulation of AMPA receptor GluR1 subunit surface expression by a 4. 1N-linked actin cytoskeletal association. J. Neurosci. 20, 7932–7940 (2000)
Coleman, S. K., Cai, C., Mottershead, D. G., Haapalahti, J. P. & Keinanen, K. Surface expression of GluR-D AMPA receptor is dependent on an interaction between its C-terminal domain and a 4.1 protein. J. Neurosci. 23, 798–806 (2003)
Jackson, A. C. & Nicoll, R. A. The expanding social network of ionotropic glutamate receptors: TARPs and other transmembrane auxiliary subunits. Neuron 70, 178–199 (2011)
Tomita, S. et al. Stargazin modulates AMPA receptor gating and trafficking by distinct domains. Nature 435, 1052–1058 (2005)
Lin, D. T. et al. Regulation of AMPA receptor extrasynaptic insertion by 4.1N, phosphorylation and palmitoylation. Nature Neurosci. 12, 879–887 (2009)
Greger, I. H., Ziff, E. B. & Penn, A. C. Molecular determinants of AMPA receptor subunit assembly. Trends Neurosci. 30, 407–416 (2007)
Contractor, A., Mulle, C. & Swanson, G. T. Kainate receptors coming of age: milestones of two decades of research. Trends Neurosci. 34, 154–163 (2011)
Zhang, W. et al. A transmembrane accessory subunit that modulates kainate-type glutamate receptors. Neuron 61, 385–396 (2009)
Copits, B. A., Robbins, J. S., Frausto, S. & Swanson, G. T. Synaptic targeting and functional modulation of GluK1 kainate receptors by the auxiliary neuropilin and tolloid-like (NETO) proteins. J. Neurosci. 31, 7334–7340 (2011)
Dargan, S. L. et al. ACET is a highly potent and specific kainate receptor antagonist: characterisation and effects on hippocampal mossy fibre function. Neuropharmacology 56, 121–130 (2009)
Lee, H. K. et al. Phosphorylation of the AMPA receptor GluR1 subunit is required for synaptic plasticity and retention of spatial memory. Cell 112, 631–643 (2003)
Makino, Y., Johnson, R. C., Yu. Y, Takamiya K & Huganir R .L Enhanced synaptic plasticity in mice with phosphomimetic mutation of the GluA1 AMPA receptor. Proc. Natl Acad. Sci. USA 108, 8450–8455 (2011)
Lisman, J., Yasuda, R. & Raghavachari, S. Mechanisms of CaMKII action in long-term potentiation. Nature Rev. Neurosci. 13, 169–182 (2012)
Opazo, P. & Choquet, D. A three-step model for the synaptic recruitment of AMPA receptors. Mol. Cell. Neurosci. 46, 1–8 (2011)
Matsuzaki, M., Honkura, N., Ellis-Davies, G. C. & Kasai, H. Structural basis of long-term potentiation in single dendritic spines. Nature 429, 761–766 (2004)
Murakoshi, H. & Yasuda, R. Postsynaptic signaling during plasticity of dendritic spines. Trends Neurosci. 35, 135–143 (2012)
Patterson, M. & Yasuda, R. Signalling pathways underlying structural plasticity of dendritic spines. Br. J. Pharmacol. 163, 1626–1638 (2011)
Stoppini, L., Buchs, P. A. & Muller, D. A simple method for organotypic cultures of nervous tissue. J. Neurosci. Methods 37, 173–182 (1991)
Schnell, E. et al. Direct interactions between PSD-95 and stargazin control synaptic AMPA receptor number. Proc. Natl Acad. Sci. USA 99, 13902–13907 (2002)
Elias, G. M., Elias, L. A., Apostolides, P. F., Kriegstein, A. R. & Nicoll, R. A. Differential trafficking of AMPA and NMDA receptors by SAP102 and PSD-95 underlies synapse development. Proc. Natl Acad. Sci. USA 105, 20953–20958 (2008)
Navarro-Quiroga, I., Chittajallu, R., Gallo, V. & Haydar, T. F. Long-term, selective gene expression in developing and adult hippocampal pyramidal neurons using focal in utero electroporation. J. Neurosci. 27, 5007–5011 (2007)
Acknowledgements
We thank A. Jackson, J. Levy, S. Fischbach, K. Lovero, N. Sheng, S. Shipman and M. Younger for critical discussions and reading of the manuscript; K. Bjorgen for technical help with organotypic slice cultures; and L. Subramanian from the Kriegstein laboratory for technical help with in utero electroporations. We thank P. Seeburg and R. Sprengel for the Gria1–3fl/fl mice. A.J.G. was supported by the National Science Foundation Graduate Research Fellowship. R.A.N. is supported by the National Institute of Health.
Author information
Authors and Affiliations
Contributions
M.C. carried out electroporations and maintained Gria1–3fl/fl mice. Y.S. collected GluK1 overexpression data. W.L. was involved in study design and cloned several constructs. A.J.G. designed the study, collected and analysed data, and wrote the paper. R.A.N. conceived the study, contributed to the design of experiments and wrote the paper. All authors discussed the results and commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
This file contains a Supplementary Discussion, Supplementary Methods, Supplementary Figures 1-9 and Supplementary References. (PDF 792 kb)
Rights and permissions
About this article
Cite this article
Granger, A., Shi, Y., Lu, W. et al. LTP requires a reserve pool of glutamate receptors independent of subunit type. Nature 493, 495–500 (2013). https://doi.org/10.1038/nature11775
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature11775
This article is cited by
-
LTP induction by structural rather than enzymatic functions of CaMKII
Nature (2023)
-
Cerebellar glutamatergic system impacts spontaneous motor recovery by regulating Gria1 expression
npj Regenerative Medicine (2022)
-
Schizophrenia-associated SAP97 mutations increase glutamatergic synapse strength in the dentate gyrus and impair contextual episodic memory in rats
Nature Communications (2022)
-
The Role of AMPARs Composition and Trafficking in Synaptic Plasticity and Diseases
Cellular and Molecular Neurobiology (2022)
-
Pharmacotherapy of Alzheimer’s disease: an overview of systematic reviews
European Journal of Clinical Pharmacology (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.