Abstract
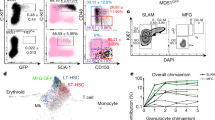
Characterization of how the microenvironment, or niche, regulates stem cell activity is central to understanding stem cell biology and to developing strategies for the therapeutic manipulation of stem cells1. Low oxygen tension (hypoxia) is commonly thought to be a shared niche characteristic in maintaining quiescence in multiple stem cell types2,3,4. However, support for the existence of a hypoxic niche has largely come from indirect evidence such as proteomic analysis5, expression of hypoxia inducible factor-1α (Hif-1α) and related genes6, and staining with surrogate hypoxic markers (for example, pimonidazole)6,7,8. Here we perform direct in vivo measurements of local oxygen tension () in the bone marrow of live mice. Using two-photon phosphorescence lifetime microscopy, we determined the absolute of the bone marrow to be quite low (<32 mm Hg) despite very high vascular density. We further uncovered heterogeneities in local , with the lowest (∼9.9 mm Hg, or 1.3%) found in deeper peri-sinusoidal regions. The endosteal region, by contrast, is less hypoxic as it is perfused with small arteries that are often positive for the marker nestin. These values change markedly after radiation and chemotherapy, pointing to the role of stress in altering the stem cell metabolic microenvironment.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Change history
10 April 2014
Gene Hif-1 was corrected to Hif-1α in the first paragraph.
References
Lymperi, S., Ferraro, F. & Scadden, D. T. The HSC niche concept has turned 31. Ann. NY Acad. Sci. 1192, 12–18 (2010)
Suda, T., Takubo, K. & Semenza, G. L. Metabolic regulation of hematopoietic stem cells in the hypoxic niche. Cell Stem Cell 9, 298–310 (2011)
Mohyeldin, A., Garzón-Muvdi, T. & Quiñones-Hinojosa, A. Oxygen in stem cell biology: a critical component of the stem cell niche. Cell Stem Cell 7, 150–161 (2010)
Lee, K. E. & Simon, M. C. From stem cells to cancer stem cells: HIF takes the stage. Curr. Opin. Cell Biol. 24, 232–235 (2012)
Unwin, R. D. Quantitative proteomics reveals posttranslational control as a regulatory factor in primary hematopoietic stem cells. Blood 107, 4687–4694 (2006)
Takubo, K., Goda, N., Yamada, W., Iriuchishima, H. & Ikeda, E. Regulation of the HIF-1α level is essential for hematopoietic stem cells. Cell Stem Cell 7, 391–402 (2010)
Ceradini, D. J. et al. Progenitor cell trafficking is regulated by hypoxic gradients through HIF-1 induction of SDF-1. Nature Med. 10, 858–864 (2004)
Parmar, K., Mauch, P., Vergilio, J.-A., Sackstein, R. & Down, J. D. Distribution of hematopoietic stem cells in the bone marrow according to regional hypoxia. Proc. Natl Acad. Sci. USA 104, 5431–5436 (2007)
Méndez-Ferrer, S. et al. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature 466, 829–834 (2010)
Kiel, M. J. et al. SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell 121, 1109–1121 (2005)
Calvi, L. M. et al. Osteoblastic cells regulate the haematopoietic stem cell niche. Nature 425, 841–846 (2003)
Wang, L. D. L. & Wagers, A. J. A. Dynamic niches in the origination and differentiation of haematopoietic stem cells. Nature Rev. Mol. Cell Biol. 12, 643–655 (2011)
Lichtman, M. A. M. The ultrastructure of the hemopoietic environment of the marrow: a review. Exp. Hematol. 9, 391–410 (1981)
Lo Celso, C. et al. Live-animal tracking of individual haematopoietic stem/progenitor cells in their niche. Nature 457, 92–96 (2009)
Nombela-Arrieta, C. et al. Quantitative imaging of haematopoietic stem and progenitor cell localization and hypoxic status in the bone marrow microenvironment. Nature Cell Biol. 15, 533–543 (2013)
Kunisaki, Y. et al. Arteriolar niches maintain haematopoietic stem cell quiescence. Nature 502, 637–643 (2013)
Chow, D. C., Wenning, L. A., Miller, W. M. & Papoutsakis, E. T. Modeling pO2 distributions in the bone marrow hematopoietic compartment. I. Krogh’s model. Biophys. J. 81, 675–684 (2001)
Chow, D. C., Wenning, L. A., Miller, W. M. & Papoutsakis, E. T. Modeling pO2 distributions in the bone marrow hematopoietic compartment. II. Modified Kroghian models. Biophys. J. 81, 685–696 (2001)
Veilleux, I., Spencer, J. A., Biss, D. P., Cote, D. & Lin, C. P. In vivo cell tracking with video rate multimodality laser scanning microscopy. IEEE J. Sel. Topics Quantum Electron. 14, 10–18 (2008)
Lebedev, A. Y., Troxler, T. & Vinogradov, S. A. Design of metalloporphyrin-based dendritic nanoprobes for two-photon microscopy of oxygen. J. Porphyr. Phthalocyanines 12, 1261–1269 (2008)
Finikova, O. S. et al. Oxygen microscopy by two-photon-excited phosphorescence. ChemPhysChem 9, 1673–1679 (2008)
Vanderkooi, J. M. J., Maniara, G. G., Green, T. J. T. & Wilson, D. F. D. An optical method for measurement of dioxygen concentration based upon quenching of phosphorescence. J. Biol. Chem. 262, 5476–5482 (1987)
Lebedev, A. Y. et al. Dendritic phosphorescent probes for oxygen imaging in biological systems. ACS Appl. Mater. Interfaces 1, 1292–1304 (2009)
Sakadžić, S. et al. Two-photon high-resolution measurement of partial pressure of oxygen in cerebral vasculature and tissue. Nature Methods 7, 755–759 (2010)
Lecoq, J. et al. Simultaneous two-photon imaging of oxygen and blood flow in deep cerebral vessels. Nature Med. 17, 893–898 (2011)
Kazmi, S. M. S. et al. Three-dimensional mapping of oxygen tension in cortical arterioles before and after occlusion. Biomed. Opt. Express 4, 1061–1073 (2013)
Dewhirst, M. W. M. et al. Quantification of longitudinal tissue pO2 gradients in window chamber tumours: impact on tumour hypoxia. Br. J. Cancer 79, 1717–1722 (1999)
Mazo, I. B. et al. Total body irradiation causes profound changes in endothelial traffic molecules for hematopoietic progenitor cell recruitment to bone marrow. Blood 99, 4182–4191 (2002)
Sipkins, D. A. et al. In vivo imaging of specialized bone marrow endothelial microdomains for tumour engraftment. Nature 435, 969–973 (2005)
Sinks, L. E. et al. Two-photon microscopy of oxygen: polymersomes as probe carrier vehicles. J. Phys. Chem. B 114, 14373–14382 (2010)
Mignone, J. L., Kukekov, V., Chiang, A. S., Steindler, D. & Enikolopov, G. Neural stem and progenitor cells in nestin-GFP transgenic mice. J. Comp. Neurol. 469, 311–324 (2004)
Esipova, T. V. et al. Two new ‘protected’ oxyphors for biological oximetry: properties and application in tumor imaging. Anal. Chem. 83, 8756–8765 (2011)
Acknowledgements
We thank S. Sakadzic for helpful discussion on setting up the 2PLM experiment. This work was supported by the US National Institutes of Health grant HL097748, EB017274 (to C.P.L.), HL097794, HL096372 and EB014703 (to D.T.S.).
Author information
Authors and Affiliations
Contributions
J.A.S. designed and built the microscope, designed experiments, conducted research, collected and analysed data and wrote the manuscript; F.F. designed experiments, conducted research, collected and analysed data, and wrote the manuscript; E.R. synthesized the PtP-C343 oxygen probe; A.K. helped conduct research and collected and analysed data; J.W., J.M.R., W.Z., L.J.M., R.T. and R.Y. helped conduct research; C.A. and D.C. helped build the microscope; S.A.V. synthesized the PtP-C343 oxygen probe and wrote the manuscript; D.T.S. designed experiments and wrote the manuscript; C.P.L. designed experiments, sponsored the project and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Extended data figures and tables
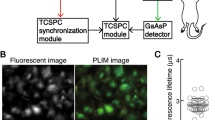
Extended Data Figure 1 Schematic of the two-photon intravital imaging and 2PLM system.
BP, bandpass filter; DAQ, data acquisition device; f, focal length; Galvo, galvanometer mirror; L, lens; LP, longpass filter; PBS, polarizing beamsplitter; PMT, photomultiplier tube; λ/2, half-wave plate. L13 and L14 have a focal length of 5 cm.
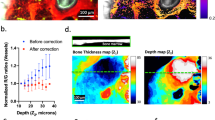
Extended Data Figure 2 In vitro calibration measurements.
The values of a solution of PtP-C343 are plotted as a function of the phosphorescent lifetime measurements recorded by our microscope. was determined by an oxygen microsensor inserted into the solution. The solid line is the published calibration curve for the same batch of PtP-C343 recorded by a different laboratory24.
Extended Data Figure 3 Schematic of PtP-C343 platinum porphyrin oxygen sensor.
The core porphyrin, PEG chains, C343 moieties and dendrimer are denoted in red, green and blue, respectively.
Extended Data Figure 4 PtP-C343 leakage into extravascular space within the BM.
Two-photon intravital image of PtP-C343 fluorescence ∼60 min after intravenous administration. Scale bar, ∼100 µm.
Extended Data Figure 5 HSC homing to bone marrow.
Scatter plot of HSC (lineagelowc-kit+Sca-1+CD150+CD48−) homing to nestin+ vessels and bone (endosteum) in lethally irradiated recipients (9.5 Gy) 2 days after adoptive transfer (n = 19 cells from 3 mice).
Extended Data Figure 6 Vascular leakage after cytoreductive conditioning.
a–c, Intravital images of BM in untreated controls (a), lethally irradiated (b) and busulphan-treated (c) mice 2 days after conditioning reveals increased permeability and decreased contrast of BM vessels. Rhodamine B–dextran (red) and bone SHG (blue) signal is shown. Scale bar, ∼50 µm.
Extended Data Figure 7 In vivo immunostaining and blood flow analysis after cytoreductive conditioning.
a, Intravital BM image of anti-Sca-1 immunostaining (red), nestin-GFP (green), blood vessels (blue) and bone (SHG, grey) demonstrating Sca-1+ nestin-GFP vessels. Scale bar, ∼50 µm. b, Standard deviation image from 600 frames of a confocal video sequence showing the path of RBC flow (green) and bone signal (SHG, blue) on day 2 after lethal irradiation (9.5 Gy). Each pixel displays the standard deviation of the corresponding pixel location across all 600 frames of the video. Scale bar, ∼100 µm.
Extended Data Figure 8 Cytoreduction of the BM after myeloablative or myelosuppressive conditioning.
BM cellularity as a function of treatment day is plotted for untreated (n = 3 mice), sub-lethally irradiated (4.5 Gy, n = 3 mice per time point), lethally irradiated (9.5 Gy, n = 3 mice per time point) and busulphan-treated (n = 3 mice per time point) mice.
Extended Data Figure 9 Revised model of local oxygen tension in the BM.
Instead of a poorly perfused hypoxic zone near the endosteum, we detected an opposite gradient, with the peri-sinusoidal region being more hypoxic than the endosteal region, which is perfused with small arteries. After irradiation, the sinusoids are greatly dilated and the blood flow is maintained, whereas the reduced oxygen consumption due to declining BM cellularity causes the to become elevated in the entire marrow space, with no defined spatial gradients.
Supplementary information
Intravital video (30 frames/sec) of blood flow in lethally irradiated mouse from Extended Data Figure 7b.
Intravital video (30 frames/sec) of blood flow in lethally irradiated mouse from Extended Data Figure 7b.
Rights and permissions
About this article
Cite this article
Spencer, J., Ferraro, F., Roussakis, E. et al. Direct measurement of local oxygen concentration in the bone marrow of live animals. Nature 508, 269–273 (2014). https://doi.org/10.1038/nature13034
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature13034
This article is cited by
-
Comparison of ex vivo bioluminescence imaging, Alu-qPCR and histology for the quantification of spontaneous lung and bone metastases in subcutaneous xenograft mouse models
Clinical & Experimental Metastasis (2024)
-
Hypoxia-cleavable and specific targeted nanomedicine delivers epigenetic drugs for enhanced treatment of breast cancer and bone metastasis
Journal of Nanobiotechnology (2023)
-
Breast cancer cells that preferentially metastasize to lung or bone are more glycolytic, synthesize serine at greater rates, and consume less ATP and NADPH than parent MDA-MB-231 cells
Cancer & Metabolism (2023)
-
The endocannabinoid system promotes hepatocyte progenitor cell proliferation and maturation by modulating cellular energetics
Cell Death Discovery (2023)
-
The functional and molecular impact of triamcinolone acetonide on primary human bone marrow mesenchymal stem cells
Scientific Reports (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.