Abstract
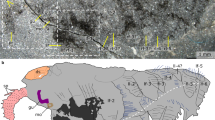
Despite being among the most celebrated taxa from Cambrian biotas, anomalocaridids (order Radiodonta) have provoked intense debate about their affinities within the moulting-animal clade that includes Arthropoda. Current alternatives identify anomalocaridids as either stem-group euarthropods1,2,3, crown-group euarthropods near the ancestry of chelicerates4, or a segmented ecdysozoan lineage with convergent similarity to arthropods in appendage construction5. Determining unambiguous affinities has been impeded by uncertainties about the segmental affiliation of anomalocaridid frontal appendages. These structures are variably homologized with jointed appendages of the second (deutocerebral) head segment, including antennae and ‘great appendages’ of Cambrian arthropods, or with the paired antenniform frontal appendages of living Onychophora and some Cambrian lobopodians. Here we describe Lyrarapax unguispinus, a new anomalocaridid from the early Cambrian Chengjiang biota, southwest China, nearly complete specimens of which preserve traces of muscles, digestive tract and brain. The traces of brain provide the first direct evidence for the segmental composition of the anomalocaridid head and its appendicular organization. Carbon-rich areas in the head resolve paired pre-protocerebral ganglia at the origin of paired frontal appendages. The ganglia connect to areas indicative of a bilateral pre-oral brain that receives projections from the eyestalk neuropils and compound retina. The dorsal, segmented brain of L. unguispinus reinforces an alliance between anomalocaridids and arthropods rather than cycloneuralians. Correspondences in brain organization between anomalocaridids and Onychophora resolve pre-protocerebral ganglia, associated with pre-ocular frontal appendages, as characters of the last common ancestor of euarthropods and onychophorans. A position of Radiodonta on the euarthropod stem-lineage implies the transformation of frontal appendages to another structure in crown-group euarthropods, with gene expression and neuroanatomy providing strong evidence that the paired, pre-oral labrum is the remnant of paired frontal appendages1.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Accession codes
Data deposits
This published work and the nomenclatural acts it contains have been deposited in ZooBank under accession number http://zoobank.org/urn:lsid:zoobank.org:pub:189DCAFF-0DD6-49C2-BE80-E999DDF059C1.
References
Budd, G. E. A palaeontological solution to the arthropod head problem. Nature 417, 271–275 (2002)
Daley, A. C., Budd, G. E., Caron, J.-B., Edgecombe, G. D. & Collins, D. The Burgess Shale anomalocaridid Hurdia and its significance for early euarthropod evolution. Science 323, 1597–1600 (2009)
Legg, D. A., Sutton, M. D. & Edgecombe, G. D. Arthropod fossil data increase congruence of morphological and molecular phylogenies. Nature Commun. 4, 2485 (2013)
Haug, J. T., Waloszek, D., Maas, A., Liu, Y. & Haug, C. Functional morphology, ontogeny and evolution of mantis shrimp-like predators in the Cambrian. Palaeontology 55, 369–399 (2012)
Hou, X. & Bergström, J. in Originations, Radiations and Biodiversity Changes: Evidences from the Chinese Fossil Record (eds Rong, J. et al.) 139–158, 847–850 (Science Press, 2006)
Vinther, J., Stein, M., Longrich, N. R. & Harper, D. A. T. A suspension-feeding anomalocarid from the Early Cambrian. Nature 507, 496–499 (2014)
Daley, A. C. & Edgecombe, G. D. Morphology of Anomalocaris canadensis from the Burgess Shale. J. Paleontol. 88, 68–91 (2014)
Daley, A. C. & Bergström, J. The oral cone of Anomalocaris is not a classic “peytoia”. Naturwissenschaften 99, 501–504 (2012)
Paterson, J. R. et al. Acute vision in the giant Cambrian predator Anomalocaris and the origin of compound eyes. Nature 480, 237–240 (2011)
Budd, G. E. Arthropod body-plan evolution in the Cambrian with an example from anomalocaridid muscle. Lethaia 31, 197–210 (1998)
Ma, X., Hou, X., Edgecombe, G. D. & Strausfeld, N. J. Complex brain and optic lobes in an early Cambrian arthropod. Nature 490, 258–261 (2012)
Tanaka, G., Hou, X., Ma, X., Edgecombe, G. D. & Strausfeld, N. J. Chelicerate neural ground pattern in a Cambrian great appendage arthropod. Nature 17, 364–367 (2013)
Schürmann, F.-W. in Arthropod Brain: Its Evolution, Development, Structure and Function (ed. Gupta A. P. ) 159–180 (John Wiley, 1987)
Mayer, G. & Whitington, P. M. Neural development in Onychophora (velvet worms) suggests a step-wise evolution of segmentation in the nervous system of Panarthropoda. Dev. Biol. 335, 263–275 (2009)
Mayer, G. & Harzsch, S. Distribution of serotonin in the trunk of Metaperipatus blainvillei (Onychophora, Peripatopsidae): implications for the evolution of the nervous system in Arthropoda. J. Comp. Neurol. 507, 1196–1208 (2008)
Eriksson, B. J., Tait, N. N., Budd, G. E. & Akam, M. The involvement of engrailed and wingless during segmentation in the onychophoran Euperipatoides kanangrensis (Peripatopsidae: Onychophora) (Reid 1996). Dev. Genes Evol. 219, 249–264 (2009)
Strausfeld, N. J., Strausfeld, C. M., Stowe, S., Rowell, D. & Loesel, R. The organization and evolutionary implications of neuropils and their neurons in the brain of the onychophoran Euperipatoides rowelli . Arthropod Struct. Dev. 35, 169–196 (2006)
Mayer, G., Whitington, P. M., Sunnucks, P. & Pflüger, H. J. A revision of brain composition in Onychophora (velvet worms) suggests that the tritocerebrum evolved in arthropods. BMC Evol. Biol. 10, 255 (2010)
Wigglesworth, V. B. The use of osmium in the fixation and staining of tissue. Proc. R. Soc. Lond. B 147, 185–199 (1957)
Strausfeld, N. J., Strausfeld, C. M., Loesel, R., Rowell, D. & Stowe, S. Arthropod phylogeny: onychophoran brain organization suggests an archaic relationship with a chelicerate stem lineage. Proc. R. Soc. B 273, 1857–1866 (2006)
Boxshall, G. A. The evolution of arthropod limbs. Biol. Rev. Camb. Philos. Soc. 79, 253–300 (2004)
Scholtz, G. & Edgecombe, G. D. The evolution of arthropod heads: reconciling morphological, developmental and palaeontological evidence. Dev. Genes Evol. 216, 395–415 (2006)
Stein, M. A new arthropod from the Early Cambrian of north Greenland, with a ‘great appendage’-like antennula. Zool. J. Linn. Soc. 158, 477–500 (2010)
Legg, D. A. & Vannier, J. The affinities of the cosmopolitan arthropod Isoxys and its implications for the origin of arthropods. Lethaia 46, 540–550 (2013)
Browne, W. E., Price, A. L., Gerberding, M. & Patel, N. H. Stages of embryonic development in the amphipod crustacean, Parhyale hawaiensis . Genesis 42, 124–149 (2005)
Haas, M. S., Brown, S. J. & Beeman, R. W. Homeotic evidence for the appendicular origin of the labrum in Tribolium castaneum . Dev. Genes Evol. 211, 96–102 (2001)
Boyan, G. S., Williams, J. L. D., Posser, S. & Braunig, P. Morphological and molecular data argue for the labrum being non-apical, articulated, and the appendage of the intercalary segment in the locust. Arthropod Struct. Dev. 31, 65–76 (2002)
Strausfeld, N. J. Arthropod Brains: Evolution, Functional Elegance, and Historical Significance Ch 9, 427–446 (Belknap, 2012)
Acknowledgements
This work was supported by the National Natural Science Foundation of China (U1302232, 41372031 and 40962001), a Leverhulme Trust Research Project Grant (F/00 696/T), by the Center for Insect Science, University of Arizona, and by a grant from the Air Force Research Laboratory (FA86511010001) to N.J.S. We thank T. Goral for assistance with energy dispersive X-ray spectroscopy. We acknowledge A. Daley’s advice about anomalocaridid anatomy.
Author information
Authors and Affiliations
Contributions
Fossil data were analysed by all authors, all of whom contributed to the text.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Extended data figures and tables
Extended Data Figure 1 Comparison of frontal appendages of Lyrarapax and Amplectobelua.
a, L. unguispinus, YKLP 13304a. b–d, Immature specimens of A. symbrachiata. Scale bars: a, 2.5 mm; b–d, 2 mm.
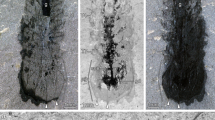
Extended Data Figure 2 Eyes of L. unguispinus and onychophoran brain.
a, Horizontal aspect of right eye of YKLP 13304b. The fracture plane has exposed radiating reliefs suggestive of rhabdomeres (rh) in a clear zone that terminate as pale bases (rhb) within a darker area interpreted as pigment withdrawn to level of basement membrane (bm) lying above paler bluish areas corresponding to expected locations of optic neuropil (opn). Occasional lenses (le) are resolved. b, Inset of same eye in the counterpart of YKLP 13304a, reversed for direct comparison with the part, radiating reliefs arrowed (rh). c, Cartoon of a, depicting identified components in a and b. d, Left eye of YKLP 13304b, with profiles (arrowed) suggestive of lenses. Scale bars: c, 0.5 mm; d, 0.5 mm. e, Frontal ganglion (frg) of E. rowelli (Onychophora) showing its supply by frontal appendage nerve (fan). Lateral protocerebrum, lpr; optic tract, opt. Scale bars: a–c, 200 μm; d, 500 μm; e, 100 μm.
Extended Data Figure 3 L. unguispinus.
a, Dorsal view of whole specimen YKLP 13306a with intact head shield (hs), eyestalks (eys), neck (ne) and eight exposed trunk segments. Rectangles refer to insets b–h. b, Right eyestalk and retina (re). c, Enlargement of the ‘shoulder’ (sh) of greatly extended flap (fl) from first trunk segment. d, Flaps of trunk segments 6 and 7 with enlargement (in e) to show setal blades (sb) and muscle (m). f, Head shield showing characteristic oval anterior margin. g, left eye stalk (eys) of counterpart (YKLP13306b) showing brown corneal layer (o) overlying darker photoreceptor layer. Scale bars: a, 2 cm; b, 3 mm; c–g, 1 cm.
Extended Data Figure 4 Relationships of Radiodonta.
Strict consensus of 108 shortest cladograms (96 steps) under equal character weights, based on modified data set from ref. 6 (Supplementary Information).
Supplementary information
Supplementary Information
This file contains details of specimen preservation, morphological description of specimens, description of phylogenetic analysis, and supplementary references. This file was replaced on 21 July 2014. (PDF 247 kb)
Supplementary Data
Dataset of characters for phylogenetic analysis in Nexus format (editable in Mesquite or NDE). (TXT 11 kb)
Rights and permissions
About this article
Cite this article
Cong, P., Ma, X., Hou, X. et al. Brain structure resolves the segmental affinity of anomalocaridid appendages. Nature 513, 538–542 (2014). https://doi.org/10.1038/nature13486
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature13486
This article is cited by
-
The visual pathway in sea spiders (Pycnogonida) displays a simple serial layout with similarities to the median eye pathway in horseshoe crabs
BMC Biology (2022)
-
The velvet worm brain unveils homologies and evolutionary novelties across panarthropods
BMC Biology (2022)
-
Unparalleled details of soft tissues in a Cretaceous ant
BMC Ecology and Evolution (2022)
-
Ordovician opabiniid-like animals and the role of the proboscis in euarthropod head evolution
Nature Communications (2022)
-
The Chengjiang Biota inhabited a deltaic environment
Nature Communications (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.