Abstract
Haematopoiesis is a developmental cascade that generates all blood cell lineages in health and disease. This process relies on quiescent haematopoietic stem cells capable of differentiating, self renewing and expanding upon physiological demand1,2. However, the mechanisms that regulate haematopoietic stem cell homeostasis and function remain largely unknown. Here we show that the neurotrophic factor receptor RET (rearranged during transfection) drives haematopoietic stem cell survival, expansion and function. We find that haematopoietic stem cells express RET and that its neurotrophic factor partners are produced in the haematopoietic stem cell environment. Ablation of Ret leads to impaired survival and reduced numbers of haematopoietic stem cells with normal differentiation potential, but loss of cell-autonomous stress response and reconstitution potential. Strikingly, RET signals provide haematopoietic stem cells with critical Bcl2 and Bcl2l1 surviving cues, downstream of p38 mitogen-activated protein (MAP) kinase and cyclic-AMP-response element binding protein (CREB) activation. Accordingly, enforced expression of RET downstream targets, Bcl2 or Bcl2l1, is sufficient to restore the activity of Ret null progenitors in vivo. Activation of RET results in improved haematopoietic stem cell survival, expansion and in vivo transplantation efficiency. Remarkably, human cord-blood progenitor expansion and transplantation is also improved by neurotrophic factors, opening the way for exploration of RET agonists in human haematopoietic stem cell transplantation. Our work shows that neurotrophic factors are novel components of the haematopoietic stem cell microenvironment, revealing that haematopoietic stem cells and neurons are regulated by similar signals.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Trumpp, A., Essers, M. & Wilson, A. Awakening dormant haematopoietic stem cells. Nature Rev. Immunol. 10, 201–209 (2010)
Morrison, S. J. & Scadden, D. T. The bone marrow niche for haematopoietic stem cells. Nature 505, 327–334 (2014)
Yamazaki, S. et al. Nonmyelinating Schwann cells maintain hematopoietic stem cell hibernation in the bone marrow niche. Cell 147, 1146–1158 (2011)
Mendez-Ferrer, S., Lucas, D., Battista, M. & Frenette, P. S. Haematopoietic stem cell release is regulated by circadian oscillations. Nature 452, 442–447 (2008)
Lucas, D. et al. Chemotherapy-induced bone marrow nerve injury impairs hematopoietic regeneration. Nature Med. 19, 695–703 (2013)
Veiga-Fernandes, H. et al. Tyrosine kinase receptor RET is a key regulator of Peyer’s Patch organogenesis. Nature 446, 547–551 (2007)
Mulligan, L. M. RET revisited: expanding the oncogenic portfolio. Nature Rev. Cancer 14, 173–186 (2014)
Almeida, A. R. et al. RET/GFRα signals are dispensable for thymic T cell development in vivo. PLoS ONE 7, e52949 (2012)
Kiel, M. J., Yilmaz, O. H., Iwashita, T., Terhorst, C. & Morrison, S. J. SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell 121, 1109–1121 (2005)
Kim, I., He, S., Yilmaz, O. H., Kiel, M. J. & Morrison, S. J. Enhanced purification of fetal liver hematopoietic stem cells using SLAM family receptors. Blood 108, 737–744 (2006)
Patel, A. et al. Differential RET signaling pathways drive development of the enteric lymphoid and nervous systems. Sci. Signal. 5, ra55 (2012)
Winkler, I. G. et al. Positioning of bone marrow hematopoietic and stromal cells relative to blood flow in vivo: serially reconstituting hematopoietic stem cells reside in distinct nonperfused niches. Blood 116, 375–385 (2010)
Ding, L. & Morrison, S. J. Haematopoietic stem cells and early lymphoid progenitors occupy distinct bone marrow niches. Nature 495, 231–235 (2013)
Schuchardt, A., D’Agati, V., Larsson-Blomberg, L., Costantini, F. & Pachnis, V. Defects in the kidney and enteric nervous system of mice lacking the tyrosine kinase receptor Ret. Nature 367, 380–383 (1994)
de Boer, J. et al. Transgenic mice with hematopoietic and lymphoid specific expression of Cre. Eur. J. Immunol. 33, 314–325 (2003)
McMahon, K. A. et al. Mll has a critical role in fetal and adult hematopoietic stem cell self-renewal. Cell Stem Cell 1, 338–345 (2007)
Mansson, R. et al. Molecular evidence for hierarchical transcriptional lineage priming in fetal and adult stem cells and multipotent progenitors. Immunity 26, 407–419 (2007)
Terskikh, A. V., Miyamoto, T., Chang, C., Diatchenko, L. & Weissman, I. L. Gene expression analysis of purified hematopoietic stem cells and committed progenitors. Blood 102, 94–101 (2003)
Thompson, B. J. et al. Control of hematopoietic stem cell quiescence by the E3 ubiquitin ligase Fbw7. J. Exp. Med. 205, 1395–1408 (2008)
Perianayagam, M. C., Madias, N. E., Pereira, B. J. & Jaber, B. L. CREB transcription factor modulates Bcl2 transcription in response to C5a in HL-60-derived neutrophils. Eur. J. Clin. Invest. 36, 353–361 (2006)
Shukla, A. et al. Activated cAMP response element binding protein is overexpressed in human mesotheliomas and inhibits apoptosis. Am. J. Pathol. 175, 2197–2206 (2009)
Nakayama, K., Negishi, I., Kuida, K., Sawa, H. & Loh, D. Y. Targeted disruption of Bcl-2αβ in mice: occurrence of gray hair, polycystic kidney disease, and lymphocytopenia. Proc. Natl Acad. Sci. USA 91, 3700–3704 (1994)
Motoyama, N. et al. Massive cell death of immature hematopoietic cells and neurons in Bcl-x-deficient mice. Science 267, 1506–1510 (1995)
Cacalano, G. et al. GFRα1 is an essential receptor component for GDNF in the developing nervous system and kidney. Neuron 21, 53–62 (1998)
Nishino, J. et al. GFR α3, a component of the artemin receptor, is required for migration and survival of the superior cervical ganglion. Neuron 23, 725–736 (1999)
Rossi, J. et al. Retarded growth and deficits in the enteric and parasympathetic nervous system in mice lacking GFR α2, a functional neurturin receptor. Neuron 22, 243–252 (1999)
Katayama, Y. et al. Signals from the sympathetic nervous system regulate hematopoietic stem cell egress from bone marrow. Cell 124, 407–421 (2006)
Mendez-Ferrer, S. et al. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature 466, 829–834 (2010)
Spiegel, A. et al. Catecholaminergic neurotransmitters regulate migration and repopulation of immature human CD34+ cells through Wnt signaling. Nature Immunol. 8, 1123–1131 (2007)
Mombaerts, P. et al. RAG-1-deficient mice have no mature B and T lymphocytes. Cell 68, 869–877 (1992)
Uesaka, T. & Enomoto, H. Neural precursor death is central to the pathogenesis of intestinal aganglionosis in Ret hypomorphic mice. J. Neurosci. 30, 5211–5218 (2010)
Kunisaki, Y. et al. Arteriolar niches maintain haematopoietic stem cell quiescence. Nature 502, 637–643 (2014)
van de Pavert, S. A. et al. Maternal retinoids control type 3 innate lymphoid cells and set the offspring immunity. Nature 508, 123–127 (2014)
Peixoto, A. et al. CD8 single-cell gene coexpression reveals three different effector types present at distinct phases of the immune response. J. Exp. Med. 204, 1193–1205 (2007)
Acknowledgements
We thank I. Monteiro Grillo and the radiotherapy service at Hospital de Santa Maria; H. Ferreira and the service of obstetrics, gynaecology and reproductive medicine at the Hospital of Santa Maria; the Instituto de Medicina Molecular animal facility, flow cytometry unit, bioimaging unit and histology unit for technical assistance. We also thank all members of H.V.-F. laboratory for discussion. D.F.-P., S.A.-M., R.G.D. and A.R.M.A. were supported by scholarships from Fundação para a Ciência e Tecnologia, Portugal. H.V.-F. was supported by Fundação para a Ciência e Tecnologia (PTDC/SAU-MII/104931/2008), Portugal, the European Molecular Biology Organisation (Project 1648), European Research Council (Project 207057) and National Blood Foundation, USA.
Author information
Authors and Affiliations
Contributions
D.F.-P., S.A.-M., M.R.-C., I.B., R.G.D., T.B., A.R.M.A. and H.R. did experiments and data analysis; H.E. generated RetBCLxL mice; D.F.-P., S.A.-M., A.P. and H.V.-F. designed in vivo and ex vivo experiments; D.F.-P. and H.V.-F. wrote the manuscript and H.V.-F. directed the study.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Extended data figures and tables
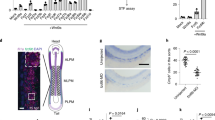
Extended Data Figure 1 Ret expression in haematopoietic progenitors and Ret ligand expression in the fetal and adult HSC environment.
a, FACS-sorted E14.5 fetal liver myeloid progenitors and LSK were analysed by RT–qPCR. b, FACS-sorted HSCs from E14.5 fetal liver and adult bone marrow were analysed by RT–qPCR. c, FACS-sorted E14.5 fetal liver and adult bone marrow HSCs were analysed by confocal microscopy. d, E14.5 fetal liver TER119−CD45−CD31+ endothelial cells (EC), TER119−CD45−CD31−cKit+ICAM-1− hepatocyte progenitor cells (HP) and TER119−CD45−CD31− cKit−ICAM-1+ mesenchymal cells (MC) were analysed by RT–qPCR (left); negative controls relative to Fig. 1c were analysed by confocal microscopy (right). e, Bone marrow TER119−CD45−CD31+Sca1+ endothelial cells (Endo), TER119−CD45−CD31−Sca1−CD51+ osteoblasts (Osteo) and TER119−CD45−CD31−Sca1+CD51+ mesenchymal stem cells (MSC) were analysed by RT–qPCR (left) and by confocal microscopy (right). f, E14.5 fetal liver and adult bone marrow were analysed by confocal microscopy. Arrows, candidate Lin−CD150+CD48−CD41− HSCs, relative to Fig. 1d, e. Figure shows negative controls for GFL staining. g, E15.5 gut tissue was analysed by confocal microscopy. White bar, 5 μm. Error bars, s.e.m. Housekeeping genes: Gapdh and Hprt1. ***P value for one-way ANOVA lower than 0.001.
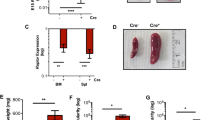
Extended Data Figure 2 LSKs are affected by Ret deficiency and have reduced reconstitution capacity.
a, E14.5 total fetal liver cells. WT n = 20; Ret−/− n = 18. b, Flow cytometry analysis of E14.5 Ret−/− and WT littermate control LSKs (top) and myeloid progenitors (bottom). c, Flow cytometry analysis of Annexin V+ cells in cultured LSK cells. d, Flow cytometry analysis of E14.5 Ret−/− and WT littermate control HSCs. e, Flow cytometry analysis of E14.5 Ret−/− and WT Lin−CD150+CD48− HSC cells. Mean fluorescence intensity of cKit was analysed. No statistically significant differences were found. f, Lin−cKit+ cells were labelled with CMTMR. Percentage of Lin−cKit+CMTMR+ cells in bone marrow and spleen 20 h after injection (n = 3). g, h, Percentage of donor CD45.2 cells in blood cell lineages 16 weeks after primary and secondary (red) transplantation, relative to Fig. 2b, d. i, Day 12 CFU-s. WT n = 10; Ret−/− n = 10. Error bars, s.e.m. *, ** and ***, P values for Student’s t-test lower than 0.05, 0.01 and 0.001 respectively.
Extended Data Figure 3 Gfra1-deficient embryos have normal LSK numbers and reconstitution potential.
a, Flow cytometry analysis of E14.5 Gfra1−/− and WT littermate control LSKs (top) and myeloid progenitors (bottom). b, Number of LSKs and total fetal liver cells. WT n = 9; Gfra1−/− n = 10. c, Gfra1−/− or WT cells were injected with a third-party CD45.1/2 competitor. Percentage of donor CD45.2 cells in blood 16 weeks after transplantation. WT n = 5; Gfra1−/− n = 3. d, Flow cytometry analysis of bone marrow LSK cells 16 weeks after transplantation. Error bars, s.e.m.
Extended Data Figure 4 Ret conditional knockout mice and analysis of haematopoietic stem cells.
a, Ret conditional knockout. Scheme of the targeted Ret allele. b, E14.5 fetal liver HSCs from Retfl littermate controls (two animals) and Ret conditional knockout Vav1-iCre.RetΔ (three animals) and deleted bone marrow control (last column) were purified by flow cytometry. Efficient deletion of Ret in Vav1-iCre.RetΔ cells was determined by qPCR as fold increase relative to littermate control cells. c, Number of E14.5 LSKs and total fetal liver cells. Retfl n = 8; RetΔ n = 8. d, Scheme of competitive transplantation with RetΔ animals and littermate controls, relative to Fig. 2h. e, Percentage of donor CD45.2 cells in blood cell lineages 16 weeks after primary and secondary transplantation. Error bars, s.e.m. *, ** and ***, P values for Student’s t-test lower than 0.05, 0.01 and 0.001 respectively.
Extended Data Figure 5 Ret expression increases after haematopoietic stress, and RET signalling increases CREB phosphorylation and cell survival.
a, Mice were sublethally irradiated. Irradiation-induced stressed HSCs were purified by flow cytometry at 72 h and analysed by RT–qPCR. b, RT–qPCR for fetal liver E14.5 Ret−/− and WT HSCs (n = 3). c, Confocal analysis of BCLxL expression in WT and Ret−/− HSCs. d, Flow cytometry analysis of Annexin V+ cells in cultured LSK cells. e, Flow cytometry of E14.5 Ret−/− and WT littermate controls. P-Akt (T308), P-S6 and PIP3: WT n = 6, Ret−/− n = 6. f, Flow cytometry analysis of LSK cells in the absence or presence of GDNF, NRTN or ARTN for 1 h (n = 6). g, Bcl2 expression in LSK cells upon GFL treatment and with different inhibitors, relative to LSKs treated with GFLs only. Light grey, isotype control. White bar, 5 μm. Error bars, s.e.m. * and **, P values for Student’s t-test lower than 0.05 and 0.01 respectively.
Extended Data Figure 6 Rescue of haematopoietic progenitors with Ret and its downstream targets.
a, Scheme of competitive transplantation, relative to Fig. 4b, c. b, Flow cytometry analysis of donor CD45.2 blood cells at 8 weeks upon transplantation of Bcl2l1-transduced WT haematopoietic progenitor cells. Error bars, s.e.m.
Extended Data Figure 7 Generation and analysis of RetBCLxL mice.
a, Ret locus was targeted by a construct containing the BCLxL coding sequence, an internal ribosomal entry site (IRES) and a puromycin resistance cassette, followed by a floxed neomycin resistance cassette to aid negative selection. Ret Bcl-xL.IRES.Puromicin knock-in mice were obtained by excising of the neomycin cassette. b, Number of myeloid progenitors, LSK cells, multipotent progenitor cells and HSCs in E14.5 fetal liver. WT n = 7; Ret+/BCLxL n = 9; RetBCLxL/BCLxL n = 8. c, FACS-sorted HSCs from Ret+/+, E14.5 fetal liver Ret+/BCLxL and adult bone marrow Ret+/BCLxL were analysed by RT–qPCR for human BCL2L1 expression. Housekeeping genes: Gapdh and Hprt1. d, Annexin V+ cells in cultured E14.5 LSK cells. WT n = 6; Ret+/BCLxL n = 4; RetBCLxL/BCLxL n = 6. e, Scheme of competitive transplantation. RetBCLxL/BCLxL animals and littermate controls were injected in competition with CD45.1/CD45.2 cells, relative to Fig. 4d, e. Error bars, s.e.m. ***P value for one-way ANOVA lower than 0.001.
Extended Data Figure 8 GFLs increase anti-apoptotic gene expression in human haematopoietic progenitors.
Human cord blood CD34+ cells were cultured in the presence or absence of GFLs for 4 days and analysed by RT–qPCR. Gene expression relative to cells cultured without GFLs. Error bars, s.e.m. ***P values for Student’s t-test lower than 0.001.
Extended Data Figure 9 Gfra2- and Gfra3-deficient embryos have normal haematopoietic progenitors.
a, Flow cytometry analysis of E14.5 Gfra2−/− and WT littermate control LSKs (top) and myeloid progenitors (bottom). b, Number of LSKs and total fetal liver cells. WT n = 12; Gfra2−/− n = 11. c, Flow cytometry analysis of E14.5 Gfra3−/− and WT littermate control LSKs (top) and myeloid progenitors (bottom). d, Number of LSKs and total fetal liver cells. WT n = 11; Gfra3−/− n = 20. Error bars, s.e.m.
Extended Data Figure 10 Neuronal growth factors regulate HSC response to physiological demand.
The neurotrophic factors GDNF, NRTN and ARTN are produced by cells in the HSC microenvironment and act directly on HSCs by activation of RET. Highlighted area: RET stimulation results in p38/MAP kinase and CREB activation leading to Bcl2 and Bcl2l1 expression. RET signals provide HSCs with survival signals that preserve HSC stemness.
Rights and permissions
About this article
Cite this article
Fonseca-Pereira, D., Arroz-Madeira, S., Rodrigues-Campos, M. et al. The neurotrophic factor receptor RET drives haematopoietic stem cell survival and function. Nature 514, 98–101 (2014). https://doi.org/10.1038/nature13498
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature13498
This article is cited by
-
Selective RET inhibitors shift the treatment pattern of RET fusion-positive NSCLC and improve survival outcomes
Journal of Cancer Research and Clinical Oncology (2023)
-
The efficacy and safety of selective RET inhibitors in RET fusion-positive non-small cell lung cancer: a meta-analysis
Investigational New Drugs (2023)
-
Cerebrolysin induces hair repigmentation associated to MART-1/Melan-A reactivation
European Journal of Medical Research (2022)
-
Maladaptive consequences of inflammatory events shape individual immune identity
Nature Immunology (2022)
-
Cholinergic signals preserve haematopoietic stem cell quiescence during regenerative haematopoiesis
Nature Communications (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.