Abstract
As a central model for morphogen action during animal development, the bone morphogenetic protein 2/4 (BMP2/4)-like ligand Decapentaplegic (Dpp) is proposed to form a long-range signalling gradient that directs both growth and pattern formation during Drosophila wing disc development1,2,3,4,5,6. While the patterning role of Dpp secreted from a stripe of cells along the anterior–posterior compartmental boundary is well established1,2,6, the mechanism by which a Dpp gradient directs uniform cell proliferation remains controversial and poorly understood7,8,9,10,11,12,13. Here, to determine the precise spatiotemporal requirements for Dpp during wing disc development, we use CRISPR–Cas9-mediated genome editing to generate a flippase recognition target (FRT)-dependent conditional null allele. By genetically removing Dpp from its endogenous stripe domain, we confirm the requirement of Dpp for the activation of a downstream phospho-Mothers against dpp (p-Mad) gradient and the regulation of the patterning targets spalt (sal), optomotor blind (omb; also known as bifid) and brinker (brk). Surprisingly, however, third-instar wing blade primordia devoid of compartmental dpp expression maintain relatively normal rates of cell proliferation and exhibit only mild defects in growth. These results indicate that during the latter half of larval development, the Dpp morphogen gradient emanating from the anterior–posterior compartment boundary is not directly required for wing disc growth.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Raftery, L. A. & Umulis, D. M. Regulation of BMP activity and range in Drosophila wing development. Curr. Opin. Cell Biol. 24, 158–165 (2012)
Affolter, M. & Basler, K. The Decapentaplegic morphogen gradient: from pattern formation to growth regulation. Nature Rev. Genet. 8, 663–674 (2007)
Lecuit, T. et al. Two distinct mechanisms for long-range patterning by Decapentaplegic in the Drosophila wing. Nature 381, 387–393 (1996)
Nellen, D., Burke, R., Struhl, G. & Basler, K. Direct and long-range action of a DPP morphogen gradient. Cell 85, 357–368 (1996)
Akiyama, T. & Gibson, M. C. Morphogen transport: theoretical and experimental controversies. Wiley Interdiscip. Rev. Dev. Biol. 4, 99–112 (2015)
Restrepo, S., Zartman, J. J. & Basler, K. Coordination of patterning and growth by the morphogen DPP. Curr. Biol. 24, R245–R255 (2014)
Rogulja, D. & Irvine, K. D. Regulation of cell proliferation by a morphogen gradient. Cell 123, 449–461 (2005)
Rogulja, D., Rauskolb, C. & Irvine, K. D. Morphogen control of wing growth through the Fat signaling pathway. Dev. Cell 15, 309–321 (2008)
Schwank, G., Restrepo, S. & Basler, K. Growth regulation by Dpp: an essential role for Brinker and a non-essential role for graded signaling levels. Development 135, 4003–4013 (2008)
Schwank, G. et al. Antagonistic growth regulation by Dpp and Fat drives uniform cell proliferation. Dev. Cell 20, 123–130 (2011)
Schwank, G., Yang, S. F., Restrepo, S. & Basler, K. Comment on “Dynamics of Dpp signaling and proliferation control”. Science 335, 401 (2012)
Wartlick, O. et al. Dynamics of Dpp signaling and proliferation control. Science 331, 1154–1159 (2011)
Day, S. J. & Lawrence, P. A. Measuring dimensions: the regulation of size and shape. Development 127, 2977–2987 (2000)
Lecuit, T. & Le Goff, L. Orchestrating size and shape during morphogenesis. Nature 450, 189–192 (2007)
Schwank, G. & Basler, K. Regulation of organ growth by morphogen gradients. Cold Spring Harb. Perspect. Biol. 2, a001669 (2010)
Kicheva, A., Bollenbach, T., Wartlick, O., Jülicher, F. & Gonzalez-Gaitan, M. Investigating the principles of morphogen gradient formation: from tissues to cells. Curr. Opin. Genet. Dev. 22, 527–532 (2012)
Müller, P., Rogers, K. W., Yu, S. R., Brand, M. & Schier, A. F. Morphogen transport. Development 140, 1621–1638 (2013)
Spencer, F. A., Hoffmann, F. M. & Gelbart, W. M. Decapentaplegic: a gene complex affecting morphogenesis in Drosophila melanogaster. Cell 28, 451–461 (1982)
Zecca, M., Basler, K. & Struhl, G. Sequential organizing activities of engrailed, hedgehog and decapentaplegic in the Drosophila wing. Development 121, 2265–2278 (1995)
St Johnston, R. D. et al. Molecular organization of the decapentaplegic gene in Drosophila melanogaster. Genes Dev. 4, 1114–1127 (1990)
Morata, G. & Ripoll, P. M inutes: mutants of Drosophila autonomously affecting cell division rate. Dev. Biol. 42, 211–221 (1975)
Domínguez, M., Wasserman, J. D. & Freeman, M. Multiple functions of the EGF receptor in Drosophila eye development. Curr. Biol. 8, 1039–1048 (1998)
Blair, S. S. Wing vein patterning in Drosophila and the analysis of intercellular signaling. Annu. Rev. Cell Dev. Biol. 23, 293–319 (2007)
Paul, L. et al. Dpp-induced Egfr signaling triggers postembryonic wing development in Drosophila. Proc. Natl Acad. Sci. USA 110, 5058–5063 (2013)
Foronda, D., Pérez-Garijo, A. & Martín, F. A. Dpp of posterior origin patterns the proximal region of the wing. Mech. Dev. 126, 99–106 (2009)
McGuire, S. E., Le, P. T., Osborn, A. J., Matsumoto, K. & Davis, R. L. Spatiotemporal rescue of memory dysfunction in Drosophila. Science 302, 1765–1768 (2003)
Xu, T. & Rubin, G. M. Analysis of genetic mosaics in developing and adult Drosophila tissues. Development 117, 1223–1237 (1993)
Kondo, S. & Ueda, R. Highly improved gene targeting by germline-specific Cas9 expression in Drosophila. Genetics 195, 715–721 (2013)
Barolo, S., Carver, L. A. & Posakony, J. W. GFP and β-galactosidase transformation vectors for promoter/enhancer analysis in Drosophila. Biotechniques 29, 726, 728, 730, 732 (2000)
Huang, A. M., Rehm, E. J. & Rubin, G. M. Quick preparation of genomic DNA from Drosophila. Cold Spring Harb. Protoc. 2009, pdb.prot5198 (2009)
Brown, T. Southern blotting. Curr. Protoc. Mol. Biol. Chapter 2, Unit2 9A (2001)
Golic, K. G. & Lindquist, S. The FLP recombinase of yeast catalyzes site-specific recombination in the Drosophila genome. Cell 59, 499–509 (1989)
Staehling-Hampton, K., Jackson, P. D., Clark, M. J., Brand, A. H. & Hoffmann, F. M. Specificity of bone morphogenetic protein-related factors: cell fate and gene expression changes in Drosophila embryos induced by decapentaplegic but not 60A. Cell Growth Differ. 5, 585–593 (1994)
Johnson, R. L., Grenier, J. K. & Scott, M. P. patched overexpression alters wing disc size and pattern: transcriptional and post-transcriptional effects on hedgehog targets. Development 121, 4161–4170 (1995)
Croker, J. A., Ziegenhorn, S. L. & Holmgren, R. A. Regulation of the Drosophila transcription factor, Cubitus interruptus, by two conserved domains. Dev. Biol. 291, 368–381 (2006)
Calleja, M., Moreno, E., Pelaz, S. & Morata, G. Visualization of gene expression in living adult Drosophila. Science 274, 252–255 (1996)
Brand, A. H. & Perrimon, N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development 118, 401–415 (1993)
Struhl, G. & Basler, K. Organizing activity of wingless protein in Drosophila. Cell 72, 527–540 (1993)
Akiyama, T. et al. Dally regulates Dpp morphogen gradient formation by stabilizing Dpp on the cell surface. Dev. Biol. 313, 408–419 (2008)
Akiyama, T., Marqués, G. & Wharton, K. A. A large bioactive BMP ligand with distinct signaling properties is produced by alternative proconvertase processing. Sci. Signal. 5, ra28 (2012)
Dejima, K., Kanai, M. I., Akiyama, T., Levings, D. C. & Nakato, H. Novel contact-dependent bone morphogenetic protein (BMP) signaling mediated by heparan sulfate proteoglycans. J. Biol. Chem. 286, 17103–17111 (2011)
Barrio, R. & de Celis, J. F. Regulation of spalt expression in the Drosophila wing blade in response to the Decapentaplegic signaling pathway. Proc. Natl Acad. Sci. USA 101, 6021–6026 (2004)
Shen, J., Dahmann, C. & Pflugfelder, G. O. Spatial discontinuity of optomotor-blind expression in the Drosophila wing imaginal disc disrupts epithelial architecture and promotes cell sorting. BMC Dev. Biol. 10, 23 (2010)
Doumpas, N. et al. Brk regulates wing disc growth in part via repression of Myc expression. EMBO Rep. 14, 261–268 (2013)
Bangi, E. & Wharton, K. Dpp and Gbb exhibit different effective ranges in the establishment of the BMP activity gradient critical for Drosophila wing patterning. Dev. Biol. 295, 178–193 (2006)
Acknowledgements
We thank G. Struhl and K. Wharton for extensive discussion and suggestions, S. Kondo for technical advice with CRISPR, K. Irvine, W. Deng and the Bloomington Stock Center for fly stocks, and R. Barrio, G. Pflugfelder, A. Teleman and the Developmental Studies Hybridoma Bank for antibodies. We thank K. Marr and L. Ellington for a critical reading of the manuscript, L. Gutchewsky for administrative support, and members of the Gibson laboratory for discussions and advice. This work was supported by funding from the Stowers Institute for Medical Research.
Author information
Authors and Affiliations
Contributions
T.A. and M.C.G. conceived the project, designed the experiments and wrote the manuscript. T.A. performed the experiments and analysed the data.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Extended data figures and tables
Extended Data Figure 1 Endogenous Dpp expression in imaginal discs.
a–f, Wing (a, b), eye–antenna (c, d), and leg (e, f) imaginal discs from UAS-GFP/+; dpp-GAL4/+ larvae are dissected and stained with anti-Dpp antibody. GFP (green) indicates dpp-GAL4-expressing cells. Note that dpp-GAL4 is not expressed in the morphogenetic furrow of the third instar eye–antenna disc (arrow in d). Dotted lines show outlines of imaginal discs. Blue: DNA. Scale bars, 100 μm. Anterior is left.
Extended Data Figure 2 Dpp and p-Mad expression in dppd12 clones.
a–h, Control (a, b, e, f) and dppd12 mutant (c, d, g, h) clones are stained with anti-Dpp (a–d) and anti-p-Mad (e–h) antibodies. Clones are marked by the absence of GFP (green). Disc boundaries are indicated by dotted lines. Scale bars, 50 μm. Anterior is to the left.
Extended Data Figure 3 FLP/FRT-mediated conditional dpp-null allele.
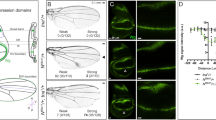
a, A flowchart describing the establishment of an FLP/FRT-mediated conditional dpp-null allele. Grey and white boxes indicate untranslated region (UTR) and dpp coding sequences, respectively. FRT sequences flank the first coding exon (exon 5). Since this exon contains the Dpp start codon and almost half of its coding sequence (the first 288/588 amino acids), FLP/FRT mediated recombination is predicted to yield a null allele. b, dppFO-GFP heterozygous, dppFO heterozygous, and dppFO homozygous adult flies. Importantly, dppFO homozygous animals have normal adult morphology.
Extended Data Figure 4 Validation of an FLP/FRT-mediated conditional allele.
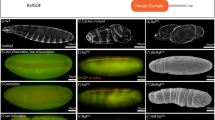
a, FRT 5′-loxP and FRT 3′ genomic regions are sequenced. b, Southern blot analysis of dppFO-GFP. Genomic DNAs from w1118 and dppFO-GFP are digested by ClaI and are subjected to Southern blot analysis using a GFP probe. c, Molecular confirmation of the FLP/FRT-mediated dpp FLP-OFF system. As expected, an FLP-OFF product (2,349 bp PCR fragment) is only detected in the dppFO/dppFO; dpp-GAL4/UAS-FLP lane. Asterisk indicates a non-specific PCR product. d, Biochemical evidence of the FLP/FRT-mediated dpp FLP-OFF system. y,w,hs-FLP; dppFO/dppFO larvae are incubated at 37 °C for 30 min at 96 h AEL to eliminate Dpp expression. After 24 h, wing discs are homogenized in SDS sample buffer and analysed by western blot analysis with anti-Dpp. Non-specific bands are indicated by an asterisk. Anti-α-tubulin is used as a loading control. e, A system to visualize the efficiency of FLP/FRT-mediated recombination. f–k, dppFO/+; dpp-GAL4/UAS-FLP,Act5c(-FRT)lacZ controls (f, g, h) and dppFO/dppFO; dpp-GAL4/UAS-FLP,Act5c(-FRT)lacZ (i, j, k) wing discs are stained with anti-Dpp and β-galactosidase antibodies. The lineage of dpp-GAL4-expressing cells is visualized by anti-β-galactosidase staining. Scale bar, 100 μm. Anterior is left.
Extended Data Figure 5 p-Mad staining of wing discs lacking dpp function in the dorsal compartment.
a–d, dppFO/ap-GAL4,UAS-GFP; UAS-FLP/+ (a, b) and dppFO/dppFO,ap-GAL4,UAS-GFP; UAS-FLP/+ (c, d) are dissected and stained with anti-p-Mad antibody. The dorso-ventral boundaries are indicated by green dotted lines. Yellow dotted lines show the disc areas. Scale bar, 50 μm.
Extended Data Figure 6 Elimination of Dpp from specific regions of wing discs.
a–f, Anti-Dpp antibody staining of wild-type (a), dppFO,ci-GAL4,en-GAL4/dppFO; UAS-FLP/+ (b), dppFO,en-GAL4/dppFO; UAS-FLP/+ (c), dppFO,ci-GAL4/dppFO; UAS-FLP/+ (d), dppFO,ap-GAL4/dppFO; UAS-FLP/+ (e), and dppFO,nub-GAL4/dppFO; UAS-FLP/+ (f). Gal4-expressing domains are highlighted in grey in each illustration. Wing disc boundaries are shown by dotted lines. Scale bar, 100 μm. Anterior is left.
Extended Data Figure 7 Spatiotemporal Dpp removal from the anterior region of wing discs.
a, A strategy for temporal Dpp elimination from the anterior compartment of wing discs using the GAL80ts system. At 18 °C, Gal4 activity is blocked by Gal80. When flies are kept at 29 °C (non-permissive temperature for GAL80ts), Gal4 induces expression of FLP and GFP. b, Timing of temperature shift. Larvae are reared at 18 °C, and are transferred to 29 °C at the indicated time points before dissection. c–f, dppFO/ci-GAL4,UAS-GFP; UAS-FLP,tub-GAL80ts/+ controls are stained with anti-Dpp. Gal4 activity is monitored by GFP expression. Scale bar, 100 μm. g, Size comparison between wing discs: dppFO/ci-GAL4,UAS-GFP; UAS-FLP,tub-GAL80ts/+ (0 (n = 21) and 72 h (n = 40) before dissection) and dppFO/dppFO,ci-GAL4,UAS-GFP; UAS-FLP,tub-GAL80ts/+ (0 (n = 36), 18 (n = 33), 24 (n = 40), 36 (n = 33), 48 (n = 80) and 72 h (n = 43) before dissection). Mean ± s.d. *P < 0.001, not significant (NS), two-sided Student’s t-test.
Rights and permissions
About this article
Cite this article
Akiyama, T., Gibson, M. Decapentaplegic and growth control in the developing Drosophila wing. Nature 527, 375–378 (2015). https://doi.org/10.1038/nature15730
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature15730
This article is cited by
-
A multiscale chemical-mechanical model predicts impact of morphogen spreading on tissue growth
npj Systems Biology and Applications (2023)
-
Lack of apoptosis leads to cellular senescence and tumorigenesis in Drosophila epithelial cells
Cell Death Discovery (2023)
-
Morphogen gradient scaling by recycling of intracellular Dpp
Nature (2022)
-
Asymmetric requirement of Dpp/BMP morphogen dispersal in the Drosophila wing disc
Nature Communications (2021)
-
Hedgehog signaling regulates regenerative patterning and growth in Harmonia axyridis leg
Cellular and Molecular Life Sciences (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.