Abstract
The integrated stress response (ISR) is a homeostatic mechanism by which eukaryotic cells sense and respond to stress-inducing signals, such as amino acid starvation. General controlled non-repressed (GCN2) kinase is a key orchestrator of the ISR, and modulates protein synthesis in response to amino acid starvation. Here we demonstrate in mice that GCN2 controls intestinal inflammation by suppressing inflammasome activation. Enhanced activation of ISR was observed in intestinal antigen presenting cells (APCs) and epithelial cells during amino acid starvation, or intestinal inflammation. Genetic deletion of Gcn2 (also known as Eif2ka4) in CD11c+ APCs or intestinal epithelial cells resulted in enhanced intestinal inflammation and T helper 17 cell (TH17) responses, owing to enhanced inflammasome activation and interleukin (IL)-1β production. This was caused by reduced autophagy in Gcn2−/− intestinal APCs and epithelial cells, leading to increased reactive oxygen species (ROS), a potent activator of inflammasomes1. Thus, conditional ablation of Atg5 or Atg7 in intestinal APCs resulted in enhanced ROS and TH17 responses. Furthermore, in vivo blockade of ROS and IL-1β resulted in inhibition of TH17 responses and reduced inflammation in Gcn2−/− mice. Importantly, acute amino acid starvation suppressed intestinal inflammation via a mechanism dependent on GCN2. These results reveal a mechanism that couples amino acid sensing with control of intestinal inflammation via GCN2.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Zhou, R., Yazdi, A. S., Menu, P. & Tschopp, J. A role for mitochondria in NLRP3 inflammasome activation. Nature 469, 221–225 (2011)
Kawai, T. & Akira, S. Toll-like receptors and their crosstalk with other innate receptors in infection and immunity. Immunity 34, 637–650 (2011)
Pulendran, B. The varieties of immunological experience: of pathogens, stress, and dendritic cells. Annu. Rev. Immunol. 33, 563–606 (2015)
Donnelly, N., Gorman, A. M., Gupta, S. & Samali, A. The eIF2α kinases: their structures and functions. Cell. Mol. Life Sci. 70, 3493–3511 (2013)
Han, A. P. et al. Heme-regulated eIF2α kinase (HRI) is required for translational regulation and survival of erythroid precursors in iron deficiency. EMBO J. 20, 6909–6918 (2001)
Querec, T. D. et al. Systems biology approach predicts immunogenicity of the yellow fever vaccine in humans. Nature Immunol. 10, 116–125 (2009)
Ravindran, R. et al. Vaccine activation of the nutrient sensor GCN2 in dendritic cells enhances antigen presentation. Science 343, 313–317 (2014)
Funke, B. et al. Functional characterisation of decoy receptor 3 in Crohn’s disease. Gut 58, 483–491 (2009)
Kugathasan, S. et al. Loci on 20q13 and 21q22 are associated with pediatric-onset inflammatory bowel disease. Nature Genet. 40, 1211–1215 (2008)
Kleene, S. J., Toews, M. L. & Adler, J. Isolation of glutamic acid methyl ester from an Escherichia coli membrane protein involved in chemotaxis. J. Biol. Chem. 252, 3214–3218 (1977)
Harding, H. P., Zhang, Y. & Ron, D. Protein translation and folding are coupled by an endoplasmic-reticulum-resident kinase. Nature 397, 271–274 (1999)
Back, S. H. et al. Translation attenuation through eIF2α phosphorylation prevents oxidative stress and maintains the differentiated state in β cells. Cell Metab. 10, 13–26 (2009)
Cao, S. S. et al. Phosphorylation of eIF2α is dispensable for differentiation but required at a posttranscriptional level for paneth cell function and intestinal homeostasis in mice. Inflamm. Bowel Dis. 20, 712–722 (2014)
Tattoli, I. et al. Amino acid starvation induced by invasive bacterial pathogens triggers an innate host defense program. Cell Host Microbe 11, 563–575 (2012)
Saitoh, T. et al. Loss of the autophagy protein Atg16L1 enhances endotoxin-induced IL-1β production. Nature 456, 264–268 (2008)
Mizushima, N. & Kuma, A. Autophagosomes in GFP-LC3 transgenic mice. Methods Mol. Biol. 445, 119–124 (2008)
Martinez, J. et al. Molecular characterization of LC3-associated phagocytosis reveals distinct roles for Rubicon, NOX2 and autophagy proteins. Nature Cell Biol. 17, 893–906 (2015)
Damiani, C. R. et al. Oxidative stress and metabolism in animal model of colitis induced by dextran sulfate sodium. J. Gastroenterol. Hepatol. 22, 1846–1851 (2007)
Brubacher, J. L. & Bols, N. C. Chemically de-acetylated 2′,7′-dichlorodihydrofluorescein diacetate as a probe of respiratory burst activity in mononuclear phagocytes. J. Immunol. Methods 251, 81–91 (2001)
Julian, D., April, K. L., Patel, S., Stein, J. R. & Wohlgemuth, S. E. Mitochondrial depolarization following hydrogen sulfide exposure in erythrocytes from a sulfide-tolerant marine invertebrate. J. Exp. Biol. 208, 4109–4122 (2005)
Zhang, P. et al. The GCN2 eIF2α kinase is required for adaptation to amino acid deprivation in mice. Mol. Cell. Biol. 22, 6681–6688 (2002)
Hao, S. et al. Uncharged tRNA and sensing of amino acid deficiency in mammalian piriform cortex. Science 307, 1776–1778 (2005)
Anthony, T. G. et al. Preservation of liver protein synthesis during dietary leucine deprivation occurs at the expense of skeletal muscle mass in mice deleted for eIF2 kinase GCN2. J. Biol. Chem. 279, 36553–36561 (2004)
Sundrud, M. S. et al. Halofuginone inhibits TH17 cell differentiation by activating the amino acid starvation response. Science 324, 1334–1338 (2009)
Kau, A. L., Ahern, P. P., Griffin, N. W., Goodman, A. L. & Gordon, J. I. Human nutrition, the gut microbiome and the immune system. Nature 474, 327–336 (2011)
Hu, J. F. et al. Repression of hepatitis B virus (HBV) transgene and HBV-induced liver injury by low protein diet. Oncogene 15, 2795–2801 (1997)
Ariyasinghe, A. et al. Protection against malaria due to innate immunity enhanced by low-protein diet. J. Parasitol. 92, 531–538 (2006)
Oarada, M. et al. Beneficial effects of a low-protein diet on host resistance to Paracoccidioides brasiliensis in mice. Nutrition 25, 954–963 (2009)
Li, C. et al. Immunopotentiation of NKT cells by low-protein diet and the suppressive effect on tumor metastasis. Cell. Immunol. 231, 96–102 (2004)
Peng, W. et al. Surgical stress resistance induced by single amino acid deprivation requires Gcn2 in mice. Sci. Transl. Med. 4, 118ra11 (2012)
Harding, H. P. et al. Regulated translation initiation controls stress-induced gene expression in mammalian cells. Mol. Cell 6, 1099–1108 (2000)
Zhang, P. et al. The PERK eukaryotic initiation factor 2 α kinase is required for the development of the skeletal system, postnatal growth, and the function and viability of the pancreas. Mol. Cell. Biol. 22, 3864–3874 (2002)
Caton, M. L., Smith-Raska, M. R. & Reizis, B. Notch–RBP-J signaling controls the homeostasis of CD8− dendritic cells in the spleen. J. Exp. Med. 204, 1653–1664 (2007)
Madison, B. B. et al. Cis elements of the villin gene control expression in restricted domains of the vertical (crypt) and horizontal (duodenum, cecum) axes of the intestine. J. Biol. Chem. 277, 33275–33283 (2002)
Mizushima, N., Yamamoto, A., Matsui, M., Yoshimori, T. & Ohsumi, Y. In vivo analysis of autophagy in response to nutrient starvation using transgenic mice expressing a fluorescent autophagosome marker. Mol. Biol. Cell 15, 1101–1111 (2004)
Acknowledgements
This work was supported by grants from the US National Institutes of Health (NIH; grants R37 DK057665, R37 AI048638, U19 AI090023, U19 AI057266) and from the Bill & Melinda Gates Foundation to B.P. R.J.K. was supported by NIH grants R01 DK088227, R37 DK042394, R01 DK103185 and the Crohn’s & Colitis Foundation of America Senior Fellow Award 3800. We thank B. Cervasi and K. P. Gill for help with sorting and D. Levesque, D. Hampton, S. Weismann and colleagues for animal husbandry and veterinary support. We thank the Center for AIDS Research core at Yerkes Primate Research Center for help with qPCR (grant P30-AI-50409) and the Integrated Cellular Imaging Center at Winship Cancer Center, Emory University for help with confocal microscopy. We also thank the Yerkes Pathology Core for performing the immunofluorescence and histology analysis. We thank S. Virgin for his advice and review of the autophagy experiments.
Author information
Authors and Affiliations
Contributions
R.R., J.L. and B.P. designed experiments and wrote the manuscript. R.R., J.L., N.K., D.K.M., H.M., S.L. and B.L. conducted the experiments. H.I.N. and L.G. performed bioinformatics analysis of the public databases. P.H. and Y.-c.W. genotyped mice. P.S. performed the histology analysis. J.M. provided critical insight and advice about the autophagy experiments. R.J.K. provided reagents and edited the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Extended data figures and tables
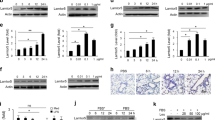
Extended Data Figure 1 eIF2α kinases are expressed in human and murine gut cells.
a, Analysis of p-eIF2α expression in APCs and epithelial cells in large intestine of naive and 2% DSS-treated mice by flow cytometry. DCs, dendritic cells; LI, large intestine. b, Comparison of immunohistological analysis of phosphorylated PKR, PERK, eIF2α and GNC2 in healthy and inflamed human colon tissue (n = 1). c, Expression levels of HRI, PKR, PERK and GCN2 in human organs quantified based on information from a public database (http://www.ebi.ac.uk). d, Expression intensity of various eIF2α kinases plotted from known published microarray data from colonic biopsies of patients with either ulcerative colitis or Crohn’s disease compared to healthy controls. Data are from one experiment that is representative of three separate experiments. *P < 0.05, **P < 0.005, ***P < 0.0005. Error bars indicate mean ± s.e.m.
Extended Data Figure 2 GCN2 deficiency does not affect the proliferation or differentiation of intestinal epithelial cells.
a–d, Immunohistology analysis (a–c) and quantification (d) of colons and jejenums from Gcn2−/− and wild-type (littermate) mice for chromagranin A (a), Ki67 (b) and lysozyme (c). KO, knockout; WT, wild type. Data are from one experiment that is representative of three separate experiments (n = 3). *P < 0.05, **P < 0.005, ***P < 0.0005. Error bars indicate mean ± s.e.m.
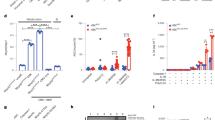
Extended Data Figure 3 GCN2 expression protects mice from DSS-induced colitis.
a, H&E staining of colon sections before and after DSS in wild-type versus Gcn2−/− mice, Gcn2fl/fl versus Gcn2Δvillin and Gcn2fl/fl versus Gcn2ΔAPC. b–d, IL-17 levels in large intestinal (b) and small intestinal (c) CD4+ T cells measured by flow cytometry; Gcn2−/− mice show increased intestinal permeability after DSS treatment as evidenced by higher levels of fluorescein isothiocyanate (FITC)-conjugated dextran in the serum (d). SI, small intestine. e, Expression of antimicrobial defensins in wild-type and Gcn2−/− mice via quantitative polymerase chain reaction (qPCR). f, IL-17 production by flow cytometry and enzyme-linked immunosorbent assay (ELISA) of OTII-CD4 T cells after culturing with different large intestinal APC subsets. Data are from one experiment that is representative of three separate experiments (n = 4–5). *P < 0.05, **P < 0.005, ***P < 0.0005. Error bars indicate mean ± s.e.m.
Extended Data Figure 4 PERK expression in epithelial cells has only a minor role in controlling mucosal homeostasis after DSS challenge.
a–d, The body weight (a), colon length (b), histology by H&E (c) and TH17 responses (d) both in the colon (large intestine; LI) and small intestine (SI) of PerkΔvillin and control wild-type littermates treated with DSS. e–h, The body weight (e), colon length (f), histology by H&E and histology score (g), and TH17 responses (h) both in the colon (LI) and small intestine (SI) of PerkΔAPC and control wild-type littermates treated with DSS. Data are representative of two separate experiments (n = 5). *P < 0.05; **P < 0.005, ***P < 0.0005. Error bars indicate mean ± s.e.m.
Extended Data Figure 5 eIF2α expression in epithelial cells and APCs control weight loss and partially control TH17 responses after DSS challenge.
a–e, The body weight (a), colon length (b), histology by H&E and histology score (c), and TH17 responses in both the colon (LI) (d) and small intestine (SI) (e) of Eif2aΔvillin and control wild-type littermates treated with DSS. f–j, The body weight (f), colon length (g), histology by H&E and histology score (h), and TH17 responses in both the colon (LI) (i) and small intestine (SI) (j) of Eif2aΔAPC and control wild-type littermates treated with DSS. Data are representative of three separate experiments (n = 5). *P < 0.05, **P < 0.005, ***P < 0.0005. Error bars indicate mean ± s.e.m.
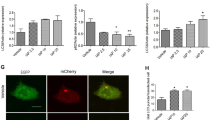
Extended Data Figure 6 Intestinal APCs and epithelial cells reveal high expression of LC3.
a, Expression of LC3–GFP in APC subsets and epithelial cells of naive LC3–GFP mice by flow cytometry (n = 3). Data are from a single experiment. b, Kinetic MFI comparison of LC3B and p62 expression with and without chloroquine on individual APC subsets and epithelial cells by flow cytometry after 12 and 24 h of single DSS administration. c, Western blot detection of LC3-I and II on lamina propria APCs before and after digitonin. d, LC3B staining of individual APCs and epithelial cells 12 h after they were treated with DSS. The portion of the lamina propria cells were subjected to digitonin before intracellular staining with the LC3B antibody. Data are representative of two separate experiments (n = 4–5). *P < 0.05, **P < 0.005, ***P < 0.0005. Error bars indicate mean ± s.e.m.
Extended Data Figure 7 Atg5 and Atg7 expression in APCs partially protects mice from DSS challenge.
a–c, Colon length (a), histology by H&E and histology score (b), and TH17 responses (c) in both the colon (LI) and small intestine (SI) of Atg5ΔAPC and control wild-type littermates treated with DSS. d–f, Colon length (d), histology by H&E and histology score (e), and TH17 responses (f) in both the colon (LI) and small intestine (SI) of Atg7ΔAPC and control wild-type littermates treated with DSS. Data are representative of three separate experiments (n = 5). *P < 0.05, **P < 0.005, ***P < 0.0005. Error bars indicate mean ± s.e.m.
Extended Data Figure 8 GCN2-induced autophagy protects the intestinal tissue from the effects of excess oxidation and inflammation.
a–f, Gcn2−/− and littermate wild-type mice were treated with 2% DSS in the drinking water for 5 days. a, MFIs of ROS and mitochondrial ROS (MitoSOX) in individual APC subsets and epithelial cells of the small intestine isolated from wild-type and Gcn2−/− mice were analysed by flow cytometry (n = 5). b, MFIs of pro-IL-1β in individual APC subsets and epithelial cells of the small intestine isolated from wild-type or Gcn2−/− mice (day 5 after DSS) were analysed by flow cytometry (n = 3). c, Histology analysis of Gcn2−/− mice that were treated with neutralizing antibody. d, Effects of low protein diet on DSS-induced colitis. e, Colon length. f, Frequencies of CD4+, CD4+IFNγ+ and CD4+Foxp3+ T cells. Data are from one experiment that is representative of two or three separate experiments. *P < 0.05, **P < 0.005, ***P < 0.0005. Error bars indicate mean ± s.e.m.
Extended Data Figure 9 Mechanisms by which GCN2 contributes to protection in the gut against acute colitis.
a, Amino acid starvation induced by an inflamed colon activates GCN2, which triggers autophagy, which is important in inhibiting oxidative stress and pro-IL-1β. Furthermore, levels of IL-1β dictate the magnitude of IL-17A-producing CD4 T cells in the colon. b, A hypothetical model for the evolutionary significance of coupling amino acid starvation with control of inflammation.
Extended Data Figure 10 Mouse phenotyping by flow cytometry and western blot.
a, b, In addition to molecular genotyping via tail DNA, we analysed the protein levels in various mucosal subsets by flow cytometry in various APC-specific (a) and epithelial-specific (b) conditional knockouts. c, Western blot to show selective depletion in APC populations in Atg5ΔAPC and Atg7ΔAPC mice. DC, dendritic cell; Ep, epithelial cell.
Rights and permissions
About this article
Cite this article
Ravindran, R., Loebbermann, J., Nakaya, H. et al. The amino acid sensor GCN2 controls gut inflammation by inhibiting inflammasome activation. Nature 531, 523–527 (2016). https://doi.org/10.1038/nature17186
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature17186
This article is cited by
-
Efficacy and safety of gut microbiota-based therapies in autoimmune and rheumatic diseases: a systematic review and meta-analysis of 80 randomized controlled trials
BMC Medicine (2024)
-
Mammalian integrated stress responses in stressed organelles and their functions
Acta Pharmacologica Sinica (2024)
-
Scribble deficiency mediates colon inflammation by inhibiting autophagy-dependent oxidative stress elimination
Scientific Reports (2023)
-
Integrative analysis of metabolomics and proteomics reveals amino acid metabolism disorder in sepsis
Journal of Translational Medicine (2022)
-
Ribosome impairment regulates intestinal stem cell identity via ZAKɑ activation
Nature Communications (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.