Abstract
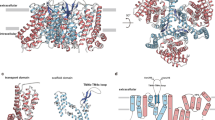
Human members of the solute carrier 1 (SLC1) family of transporters take up excitatory neurotransmitters in the brain and amino acids in peripheral organs. Dysregulation of the function of SLC1 transporters is associated with neurodegenerative disorders and cancer. Here we present crystal structures of a thermostabilized human SLC1 transporter, the excitatory amino acid transporter 1 (EAAT1), with and without allosteric and competitive inhibitors bound. The structures reveal architectural features of the human transporters, such as intra- and extracellular domains that have potential roles in transport function, regulation by lipids and post-translational modifications. The coordination of the allosteric inhibitor in the structures and the change in the transporter dynamics measured by hydrogen–deuterium exchange mass spectrometry reveal a mechanism of inhibition, in which the transporter is locked in the outward-facing states of the transport cycle. Our results provide insights into the molecular mechanisms underlying the function and pharmacology of human SLC1 transporters.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Slotboom, D. J., Konings, W. N. & Lolkema, J. S. Structural features of the glutamate transporter family. Microbiol. Mol. Biol. Rev. 63, 293–307 (1999)
Kanai, Y. & Hediger, M. A. The glutamate and neutral amino acid transporter family: physiological and pharmacological implications. Eur. J. Pharmacol. 479, 237–247 (2003)
Danbolt, N. C. Glutamate uptake. Prog. Neurobiol. 65, 1–105 (2001)
Lehre, K. P. & Danbolt, N. C. The number of glutamate transporter subtype molecules at glutamatergic synapses: chemical and stereological quantification in young adult rat brain. J. Neurosci. 18, 8751–8757 (1998)
Zerangue, N. & Kavanaugh, M. P. Flux coupling in a neuronal glutamate transporter. Nature 383, 634–637 (1996)
Rothstein, J. D., Van Kammen, M., Levey, A. I., Martin, L. J. & Kuncl, R. W. Selective loss of glial glutamate transporter GLT-1 in amyotrophic lateral sclerosis. Ann. Neurol. 38, 73–84 (1995)
Winter, N., Kovermann, P. & Fahlke, C. A point mutation associated with episodic ataxia 6 increases glutamate transporter anion currents. Brain 135, 3416–3425 (2012)
Choi, K. D. et al. Late-onset episodic ataxia associated with SLC1A3 mutation. J. Hum. Genet. 62, 443–446 (2016)
Chao, X. D., Fei, F. & Fei, Z. The role of excitatory amino acid transporters in cerebral ischemia. Neurochem. Res. 35, 1224–1230 (2010)
Pilc, A., Wieron´ska, J. M. & Skolnick, P. Glutamate-based antidepressants: preclinical psychopharmacology. Biol. Psychiatry 73, 1125–1132 (2013)
Robert, S. M. & Sontheimer, H. Glutamate transporters in the biology of malignant gliomas. Cell. Mol. Life Sci. 71, 1839–1854 (2014)
Zerangue, N. & Kavanaugh, M. P. ASCT-1 is a neutral amino acid exchanger with chloride channel activity. J. Biol. Chem. 271, 27991–27994 (1996)
Wang, Q. et al. Targeting glutamine transport to suppress melanoma cell growth. Int. J. Cancer 135, 1060–1071 (2014)
Shimizu, K. et al. ASC amino-acid transporter 2 (ASCT2) as a novel prognostic marker in non-small cell lung cancer. Br. J. Cancer 110, 2030–2039 (2014)
Wang, Q. et al. Targeting ASCT2-mediated glutamine uptake blocks prostate cancer growth and tumour development. J. Pathol. 236, 278–289 (2015)
van Geldermalsen, M. et al. ASCT2/SLC1A5 controls glutamine uptake and tumour growth in triple-negative basal-like breast cancer. Oncogene 35, 3201–3208 (2016)
Shimamoto, K. Glutamate transporter blockers for elucidation of the function of excitatory neurotransmission systems. Chem. Rec. 8, 182–199 (2008)
Grewer, C. & Grabsch, E. New inhibitors for the neutral amino acid transporter ASCT2 reveal its Na+-dependent anion leak. J. Physiol. (Lond.) 557, 747–759 (2004)
Jensen, A. A. et al. Discovery of the first selective inhibitor of excitatory amino acid transporter subtype 1. J. Med. Chem. 52, 912–915 (2009)
Abrahamsen, B. et al. Allosteric modulation of an excitatory amino acid transporter: the subtype-selective inhibitor UCPH-101 exerts sustained inhibition of EAAT1 through an intramonomeric site in the trimerization domain. J. Neurosci. 33, 1068–1087 (2013)
Yernool, D., Boudker, O., Jin, Y. & Gouaux, E. Structure of a glutamate transporter homologue from Pyrococcus horikoshii. Nature 431, 811–818 (2004)
Reyes, N., Ginter, C. & Boudker, O. Transport mechanism of a bacterial homologue of glutamate transporters. Nature 462, 880–885 (2009)
Akyuz, N. et al. Transport domain unlocking sets the uptake rate of an aspartate transporter. Nature 518, 68–73 (2015)
Boudker, O., Ryan, R. M., Yernool, D., Shimamoto, K. & Gouaux, E. Coupling substrate and ion binding to extracellular gate of a sodium-dependent aspartate transporter. Nature 445, 387–393 (2007)
Zhang, Y., Bendahan, A., Zarbiv, R., Kavanaugh, M. P. & Kanner, B. I. Molecular determinant of ion selectivity of a (Na+ + K+)-coupled rat brain glutamate transporter. Proc. Natl Acad. Sci. USA 95, 751–755 (1998)
Seal, R. P. & Amara, S. G. A reentrant loop domain in the glutamate carrier EAAT1 participates in substrate binding and translocation. Neuron 21, 1487–1498 (1998)
Tao, Z. et al. Mechanism of cation binding to the glutamate transporter EAAC1 probed with mutation of the conserved amino acid residue Thr101. J. Biol. Chem. 285, 17725–17733 (2010)
Larsson, H. P. et al. Evidence for a third sodium-binding site in glutamate transporters suggests an ion/substrate coupling model. Proc. Natl Acad. Sci. USA 107, 13912–13917 (2010)
Guskov, A., Jensen, S., Faustino, I., Marrink, S. J. & Slotboom, D. J. Coupled binding mechanism of three sodium ions and aspartate in the glutamate transporter homologue GltTk. Nat. Commun. 7, 13420 (2016)
Crisman, T. J., Qu, S., Kanner, B. I. & Forrest, L. R. Inward-facing conformation of glutamate transporters as revealed by their inverted-topology structural repeats. Proc. Natl Acad. Sci. USA 106, 20752–20757 (2009)
Focke, P. J., Moenne-Loccoz, P. & Larsson, H. P. Opposite movement of the external gate of a glutamate transporter homolog upon binding cotransported sodium compared with substrate. J. Neurosci. 31, 6255–6262 (2011)
Brocke, L., Bendahan, A., Grunewald, M. & Kanner, B. I. Proximity of two oppositely oriented reentrant loops in the glutamate transporter GLT-1 identified by paired cysteine mutagenesis. J. Biol. Chem. 277, 3985–3992 (2002)
Qu, S. & Kanner, B. I. Substrates and non-transportable analogues induce structural rearrangements at the extracellular entrance of the glial glutamate transporter GLT-1/EAAT2. J. Biol. Chem. 283, 26391–26400 (2008)
Seal, R. P., Leighton, B. H. & Amara, S. G. A model for the topology of excitatory amino acid transporters determined by the extracellular accessibility of substituted cysteines. Neuron 25, 695–706 (2000)
Grunewald, M., Bendahan, A. & Kanner, B. I. Biotinylation of single cysteine mutants of the glutamate transporter GLT-1 from rat brain reveals its unusual topology. Neuron 21, 623–632 (1998)
Ryan, R. M., Kortt, N. C., Sirivanta, T. & Vandenberg, R. J. The position of an arginine residue influences substrate affinity and K+ coupling in the human glutamate transporter, EAAT1. J. Neurochem. 114, 565–575 (2010)
Borre, L. & Kanner, B. I. Arginine 445 controls the coupling between glutamate and cations in the neuronal transporter EAAC-1. J. Biol. Chem. 279, 2513–2519 (2004)
Bailey, C. G. et al. Loss-of-function mutations in the glutamate transporter SLC1A1 cause human dicarboxylic aminoaciduria. J. Clin. Invest. 121, 446–453 (2011)
Leinenweber, A., Machtens, J. P., Begemann, B. & Fahlke, C. Regulation of glial glutamate transporters by C-terminal domains. J. Biol. Chem. 286, 1927–1937 (2011)
Shouffani, A. & Kanner, B. I. Cholesterol is required for the reconstruction of the sodium- and chloride-coupled, gamma-aminobutyric acid transporter from rat brain. J. Biol. Chem. 265, 6002–6008 (1990)
Butchbach, M. E., Tian, G., Guo, H. & Lin, C. L. Association of excitatory amino acid transporters, especially EAAT2, with cholesterol-rich lipid raft microdomains: importance for excitatory amino acid transporter localization and function. J. Biol. Chem. 279, 34388–34396 (2004)
McIlwain, B. C., Vandenberg, R. J. & Ryan, R. M. Transport rates of a glutamate transporter homologue are influenced by the lipid bilayer. J. Biol. Chem. 290, 9780–9788 (2015)
Fairman, W. A., Sonders, M. S., Murdoch, G. H. & Amara, S. G. Arachidonic acid elicits a substrate-gated proton current associated with the glutamate transporter EAAT4. Nat. Neurosci. 1, 105–113 (1998)
Raunser, S. et al. Heterologously expressed GLT-1 associates in approximately 200-nm protein-lipid islands. Biophys. J. 91, 3718–3726 (2006)
Wales, T. E. & Engen, J. R. Hydrogen exchange mass spectrometry for the analysis of protein dynamics. Mass Spectrom. Rev. 25, 158–170 (2006)
Konermann, L., Pan, J. & Liu, Y. H. Hydrogen exchange mass spectrometry for studying protein structure and dynamics. Chem. Soc. Rev. 40, 1224–1234 (2011)
Shimamoto, K. et al. Characterization of novel l-threo-β-benzyloxyaspartate derivatives, potent blockers of the glutamate transporters. Mol. Pharmacol. 65, 1008–1015 (2004)
Watzke, N. & Grewer, C. The anion conductance of the glutamate transporter EAAC1 depends on the direction of glutamate transport. FEBS Lett. 503, 121–125 (2001)
Reyes, N., Oh, S. & Boudker, O. Binding thermodynamics of a glutamate transporter homolog. Nat. Struct. Mol. Biol. 20, 634–640 (2013)
Kawate, T. & Gouaux, E. Fluorescence-detection size-exclusion chromatography for precrystallization screening of integral membrane proteins. Structure 14, 673–681 (2006)
Steipe, B., Schiller, B., Plückthun, A. & Steinbacher, S. Sequence statistics reliably predict stabilizing mutations in a protein domain. J. Mol. Biol. 240, 188–192 (1994)
Kabsch, W. Xds. Acta Crystallogr. D 66, 125–132 (2010)
Evans, P. R. & Murshudov, G. N. How good are my data and what is the resolution? Acta Crystallogr. D 69, 1204–1214 (2013)
McCoy, A. J. et al. Phaser crystallographic software. J. Appl. Crystallogr. 40, 658–674 (2007)
Emsley, P., Lohkamp, B., Scott, W. G. & Cowtan, K. Features and development of Coot. Acta Crystallogr. D 66, 486–501 (2010)
Blanc, E. et al. Refinement of severely incomplete structures with maximum likelihood in BUSTER-TNT. Acta Crystallogr. D 60, 2210–2221 (2004)
O’Brien, D. P. et al. Structural models of intrinsically disordered and calcium-bound folded states of a protein adapted for secretion. Sci. Rep. 5, 14223 (2015)
Hourdel, V. et al. MEMHDX: an interactive tool to expedite the statistical validation and visualization of large HDX-MS datasets. Bioinformatics 32, 3413–3419 (2016)
Waterhouse, A. M., Procter, J. B., Martin, D. M., Clamp, M. & Barton, G. J. Jalview Version 2—a multiple sequence alignment editor and analysis workbench. Bioinformatics 25, 1189–1191 (2009)
Acknowledgements
We thank O. Boudker for comments on the manuscript and discussion on consensus mutagenesis; P. V. Krasteva for comments on the manuscript; A. Haouz and the staff at the crystallogenesis core facility of the Institut Pasteur for assistance with crystallization screens; Staff at Synchrotron SOLEIL and the European Synchrotron Radiation Facility for assistance with data collection; D. O’Brien for discussion of HDX results. The work was funded by the ERC Starting grant 309657 (N.R.). Further support from G5 Institut Pasteur funds (N.R.), CACSICE grant (ANR-11-EQPX-008), and CNRS UMR3528 (N.R., J.C.-R.) is acknowledged.
Author information
Authors and Affiliations
Contributions
J.C.C.-T. and R.A. optimized and performed protein expression, purification and crystallization, and R.A. performed molecular biology; J.C.C.-T., R.A. and N.R. collected crystallographic data, and J.C.C.-T., P.L. and N.R. analysed diffraction data and structures; E.C. and R.A performed and analysed uptake experiments; E.C. prepared protein samples for HDX-MS; S.B. collected and analysed HDX-MS data with help from E.C.; All authors contributed to the experimental design of the project and manuscript preparation. N.R. conceived and supervised the project.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Additional information
Reviewer Information Nature thanks C. Miller and the other anonymous reviewer(s) for their contribution to the peer review of this work.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Figure 1 Alignment of human SLC1 transporters.
Amino acid sequences of EAAT1–EAAT5, ASCT1–ASCT2 and EAAT1cryst are compared. The boundaries of the α-helices (cylinders) in the TranD (orange) and ScaD (teal) seen in the EAAT1cryst structure are shown. To confer crystallizability, the region between TM3 and TM4c (arrows) from ASCT2 was transferred to a thermally stabilized EAAT1. To improve crystal formation in the absence of UPCH101, Met231Ile and Phe235Ile mutations (circles) were introduced to generate EAAT1cryst-II. These substitutions are found in EAAT2. Other residues involved in UPCH101 coordination are more conserved (triangles). Sequences were aligned with Jalview59.
Extended Data Figure 2 EAAT1cryst and EAAT1 glutamate uptake.
a, Initial rates of l-glutamate uptake from purified EAAT1cryst reconstituted in liposomes. The solid line is the fit of a Michaelis–Menten equation to the data with Km = 21 ± 10 μM and Vmax = 13 ± 1 pmol μg−1 protein min−1. The graph is the mean of three independent experiments, and error bars represent s.e.m. b, l-glutamate uptake was measured in HEK293F cells expressing wild-type EAAT1 (black circles) and a truncated mutant beyond Glu501 (red symbols). The initial rate of uptake decreased by approximately 2-fold in the EAAT1-truncated mutant. Data were normalized to the asymptotic level of glutamate uptake based on a monoexponential function. The rates obtained from the fits were 0.16 ± 0.03 min−1 and 0.08 ± 0.03 min−1 for EAAT1 and the truncated mutant, respectively. The graphs are means of four independent experiments performed in duplicate. Error bars represent the s.e.m.
Extended Data Figure 3 EAAT1cryst and GltPh structural comparison.
a, b, EAAT1cryst aligns to a monomer of GltPh (PDB code 2NWL), with a Cα root mean squared deviation (r.m.s.d.) value of 1.4 Å. The ScaDs (EAAT1cryst teal, and GltPh purple; a), and TranDs (EAAT1cryst orange, and GltPh purple; b) are shown separately for clarity of display.
Extended Data Figure 4 EAAT1cryst trimeric interface.
a, b, Interface of three ScaDs of the EAAT1cryst UCPH101-bound structure viewed from the extracellular side (a) and from the membrane (b). The TranDs are not shown. The ScaD of one monomer (black) buries 3,000 Å2 in the trimerization interface through extensive contacts with the two other subunits (teal and purple surfaces), including six intermolecular salt bridges (shown as green sticks for the monomer in black). The surface area buried at the trimeric interface in the other two monomers is coloured light pink. Only residues that contribute ≥10 Å2 of buried surface area are highlighted.
Extended Data Figure 5 TranD–ScaD interface.
a, b, EAAT1cryst monomer viewed from the membrane (solid black line). Residues in the TranD (coloured black) bury 1,760 Å2 at the interface with the ScaD (a). This interface extends to the extracellular side of the transporter through interactions between HP2–TM4 (sticks and pseudo-transparent spheres) (b). c, Cytoplasmic view of the monomer displaying the salt bridge between TM7 and TM5.
Extended Data Figure 6 Superposition of EAAT1cryst and EAAT1cryst-II structures.
a, b, The transport domains of EAAT1cryst (teal) and EAAT1cryst-II (pink) UCPH101-bound structures superimpose accurately after aligning their scaffold domains (a). The overall Cα r.m.s.d. value was 0.3 Å. However, the same alignment done with EAAT1cryst-II UCPH101-bound and -unbound structures shows a small but global movement of the transport domain (b), with a small increase in the overall Cα r.m.s.d. of 0.1 Å. c, d, Anomalous difference Fourier maps contoured at 2.8σ (pink mesh), from data collected at low energy X-rays (1.77 Å), show the correct sequence registry in both the TranD (orange, a) and the ScaD (teal, b).
Extended Data Figure 7 Peptide coverage map of EAAT1cryst.
A total of 111 peptides covering 76.3% of the EAAT1cryst sequence were identified by data-independent MS/MS acquisition after 2 min digestion with immobilized pepsin. Each bar below the EAAT1cryst sequence corresponds to a unique peptide. The 57 peptides coloured blue were further selected for HDX-MS data extraction and analysis. The two additional N-terminal residues (that is, Gly and Pro) that remain after protein purification are also shown. The transmembrane helices of the TranD (orange) and the ScaD (cyan) are indicated above the sequence.
Extended Data Figure 8 UCPH101 effect on the local hydrogen exchange behaviour of EAAT1cryst.
a, HDX profiles of EAAT1cryst (see Methods) in the apo unbound (top) and UCPH101-bound (bottom) state. The relative fractional uptake determined for each peptide and at each time point is plotted as a function of peptide position. The black to red lines correspond to data acquired from 10 s up to 1 h, respectively. b, The fractional uptake difference plot was generated by subtracting the deuterium uptake values in the UCPH101-unbound from those in the bound state. Negative uptake difference indicates an UCPH101-induced decrease in amide hydrogen exchange. Each dot corresponds to an average of three independent HDX-MS experiments. The four regions (labelled 1–4) that show a statistically significant modification (Wald test; P < 0.01) of deuterium uptake upon binding of UCPH101 are highlighted in grey.
Extended Data Figure 9 HDX-MS results mapped on the crystal structure of ScaD and TranD of EAAT1cryst in the unbound and UCPH101-bound state.
The colour code at the bottom shows the average relative fractional uptake measured in both domains after 10 s (top), 10 min (middle) and 1 h (bottom) labelling. Missing regions in the crystal structure are represented by dashed lines. Peptides that show a statistically significant (Wald test; P < 0.01) modification of deuterium uptake upon UCPH101 binding are labelled. Uncovered regions are coloured light blue.
Rights and permissions
About this article
Cite this article
Canul-Tec, J., Assal, R., Cirri, E. et al. Structure and allosteric inhibition of excitatory amino acid transporter 1. Nature 544, 446–451 (2017). https://doi.org/10.1038/nature22064
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature22064
This article is cited by
-
Evidence for transporter-mediated uptake of environmental l-glutamate in a freshwater sponge, Ephydatia muelleri
Journal of Comparative Physiology B (2024)
-
Neuronal DSCAM regulates the peri-synaptic localization of GLAST in Bergmann glia for functional synapse formation
Nature Communications (2024)
-
Symport and antiport mechanisms of human glutamate transporters
Nature Communications (2023)
-
Fatty acid transporter MFSD2A is a multifunctional gatekeeper in brain and placenta
Nature Structural & Molecular Biology (2022)
-
Structural basis of sodium-dependent bile salt uptake into the liver
Nature (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.