Abstract
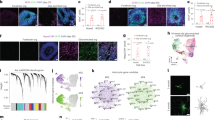
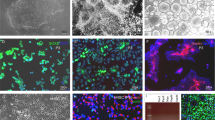
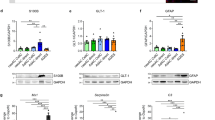
Human pluripotent stem cells (hPSCs) have been differentiated efficiently to neuronal cell types. However, directed differentiation of hPSCs to astrocytes and astroglial subtypes remains elusive. In this study, hPSCs were directed to nearly uniform populations of immature astrocytes (>90% S100β+ and GFAP+) in large quantities. The immature human astrocytes exhibit similar gene expression patterns as primary astrocytes, display functional properties such as glutamate uptake and promotion of synaptogenesis, and become mature astrocytes by forming connections with blood vessels after transplantation into the mouse brain. Furthermore, hPSC-derived neuroepithelia, patterned to rostral-caudal and dorsal-ventral identities with the same morphogens used for neuronal subtype specification, generate immature astrocytes that express distinct homeodomain transcription factors and display phenotypic differences of different astroglial subtypes. These human astroglial progenitors and immature astrocytes will be useful for studying astrocytes in brain development and function, understanding the roles of astrocytes in disease processes and developing novel treatments for neurological disorders.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Barres, B.A. The mystery and magic of glia: a perspective on their roles in health and disease. Neuron 60, 430–440 (2008).
Kettenmann, H. & Verkhratsky, A. Neuroglia: the 150 years after. Trends Neurosci. 31, 653–659 (2008).
Zhang, S.C. Defining glial cells during CNS development. Nat. Rev. Neurosci. 2, 840–843 (2001).
Rowitch, D.H. & Kriegstein, A.R. Developmental genetics of vertebrate glial-cell specification. Nature 468, 214–222 (2010).
Ullian, E.M., Sapperstein, S.K., Christopherson, K.S. & Barres, B.A. Control of synapse number by glia. Science 291, 657–661 (2001).
Johnson, M.A., Weick, J.P., Pearce, R.A. & Zhang, S.C. Functional neural development from human embryonic stem cells: accelerated synaptic activity via astrocyte coculture. J. Neurosci. 27, 3069–3077 (2007).
Rothstein, J.D. et al. Knockout of glutamate transporters reveals a major role for astroglial transport in excitotoxicity and clearance of glutamate. Neuron 16, 675–686 (1996).
Iadecola, C. & Nedergaard, M. Glial regulation of the cerebral microvasculature. Nat. Neurosci. 10, 1369–1376 (2007).
Rouach, N., Koulakoff, A., Abudara, V., Willecke, K. & Giaume, C. Astroglial metabolic networks sustain hippocampal synaptic transmission. Science 322, 1551–1555 (2008).
Oberheim, N.A. et al. Loss of astrocytic domain organization in the epileptic brain. J. Neurosci. 28, 3264–3276 (2008).
Brenner, M. et al. Mutations in GFAP, encoding glial fibrillary acidic protein, are associated with Alexander disease. Nat. Genet. 27, 117–120 (2001).
Seifert, G., Schilling, K. & Steinhauser, C. Astrocyte dysfunction in neurological disorders: a molecular perspective. Nat. Rev. Neurosci. 7, 194–206 (2006).
Lobsiger, C.S. & Cleveland, D.W. Glial cells as intrinsic components of non-cell-autonomous neurodegenerative disease. Nat. Neurosci. 10, 1355–1360 (2007).
Emsley, J.G. & Macklis, J.D. Astroglial heterogeneity closely reflects the neuronal-defined anatomy of the adult murine CNS. Neuron Glia Biol. 2, 175–186 (2006).
Bachoo, R.M. et al. Molecular diversity of astrocytes with implications for neurological disorders. Proc. Natl. Acad. Sci. USA 101, 8384–8389 (2004).
Yeh, T.H., Lee da, Y., Gianino, S.M. & Gutmann, D.H. Microarray analyses reveal regional astrocyte heterogeneity with implications for neurofibromatosis type 1 (NF1)-regulated glial proliferation. Glia 57, 1239–1249 (2009).
Guatteo, E., Stanness, K.A. & Janigro, D. Hyperpolarization-activated ion currents in cultured rat cortical and spinal cord astrocytes. Glia 16, 196–209 (1996).
Blomstrand, F., Aberg, N.D., Eriksson, P.S., Hansson, E. & Ronnback, L. Extent of intercellular calcium wave propagation is related to gap junction permeability and level of connexin-43 expression in astrocytes in primary cultures from four brain regions. Neuroscience 92, 255–265 (1999).
Haas, B. et al. Activity-dependent ATP-waves in the mouse neocortex are independent from astrocytic calcium waves. Cereb. Cortex 16, 237–246 (2006).
Muroyama, Y., Fujiwara, Y., Orkin, S.H. & Rowitch, D.H. Specification of astrocytes by bHLH protein SCL in a restricted region of the neural tube. Nature 438, 360–363 (2005).
Sugimori, M. et al. Combinatorial actions of patterning and HLH transcription factors in the spatiotemporal control of neurogenesis and gliogenesis in the developing spinal cord. Development 134, 1617–1629 (2007).
Hochstim, C., Deneen, B., Lukaszewicz, A., Zhou, Q. & Anderson, D.J. Identification of positionally distinct astrocyte subtypes whose identities are specified by a homeodomain code. Cell 133, 510–522 (2008).
O'Leary, D.D., Chou, S.J. & Sahara, S. Area patterning of the mammalian cortex. Neuron 56, 252–269 (2007).
Niederreither, K. & Dolle, P. Retinoic acid in development: towards an integrated view. Nat. Rev. Genet. 9, 541–553 (2008).
Cahoy, J.D. et al. A transcriptome database for astrocytes, neurons, and oligodendrocytes: a new resource for understanding brain development and function. J. Neurosci. 28, 264–278 (2008).
Nishiyama, A., Yang, Z. & Butt, A. Astrocytes and NG2-glia: what's in a name? J. Anat. 207, 687–693 (2005).
Liu, Y. et al. CD44 expression identifies astrocyte-restricted precursor cells. Dev. Biol. 276, 31–46 (2004).
Deneen, B. et al. The transcription factor NFIA controls the onset of gliogenesis in the developing spinal cord. Neuron 52, 953–968 (2006).
Wilkinson, M., Hume, R., Strange, R. & Bell, J.E. Glial and neuronal differentiation in the human fetal brain 9–23 weeks of gestation. Neuropathol. Appl. Neurobiol. 16, 193–204 (1990).
Pal, U., Chaudhury, S. & Sarkar, P.K. Tubulin and glial fibrillary acidic protein gene expression in developing fetal human brain at midgestation. Neurochem. Res. 24, 637–641 (1999).
Hu, B.Y. et al. Neural differentiation of human induced pluripotent stem cells follows developmental principles but with variable potency. Proc. Natl. Acad. Sci. USA 107, 4335–4340 (2010).
Pankratz, M.T. et al. Directed neural differentiation of human embryonic stem cells via an obligated primitive anterior stage. Stem Cells 25, 1511–1520 (2007).
Li, X.J. et al. Coordination of sonic hedgehog and Wnt signaling determines ventral and dorsal telencephalic neuron types from human embryonic stem cells. Development 136, 4055–4063 (2009).
Zhou, M., Schools, G.P. & Kimelberg, H.K. Development of GLAST+ astrocytes and NG2+ glia in rat hippocampus CA1: mature astrocytes are electrophysiologically passive. J. Neurophysiol. 95, 134–143 (2006).
Scemes, E. & Giaume, C. Astrocyte calcium waves: what they are and what they do. Glia 54, 716–725 (2006).
Doengi, M. et al. GABA uptake-dependent Ca2+ signaling in developing olfactory bulb astrocytes. Proc. Natl. Acad. Sci. USA 106, 17570–17575 (2009).
Christopherson, K.S. et al. Thrombospondins are astrocyte-secreted proteins that promote CNS synaptogenesis. Cell 120, 421–433 (2005).
Simard, M., Arcuino, G., Takano, T., Liu, Q.S. & Nedergaard, M. Signaling at the gliovascular interface. J. Neurosci. 23, 9254–9262 (2003).
Guillaume, D.J., Johnson, M.A., Li, X.J. & Zhang, S.C. Human embryonic stem cell-derived neural precursors develop into neurons and integrate into the host brain. J. Neurosci. Res. 84, 1165–1176 (2006).
Oberheim, N.A. et al. Uniquely hominid features of adult human astrocytes. J. Neurosci. 29, 3276–3287 (2009).
Matyash, V. & Kettenmann, H. Heterogeneity in astrocyte morphology and physiology. Brain Res. Rev. 63, 2–10 (2010).
Rowitch, D.H. Glial specification in the vertebrate neural tube. Nat. Rev. Neurosci. 5, 409–419 (2004).
Kessaris, N., Pringle, N. & Richardson, W.D. Specification of CNS glia from neural stem cells in the embryonic neuroepithelium. Phil. Trans. R. Soc. Lond. B 363, 71–85 (2008).
Hewett, J.A. Determinants of regional and local diversity within the astroglial lineage of the normal central nervous system. J. Neurochem. 110, 1717–1736 (2009).
Silver, D.J. & Steindler, D.A. Common astrocytic programs during brain development, injury and cancer. Trends Neurosci. 32, 303–311 (2009).
Lepore, A.C. et al. Focal transplantation-based astrocyte replacement is neuroprotective in a model of motor neuron disease. Nat. Neurosci. 11, 1294–1301 (2008).
Yu, J. et al. Induced pluripotent stem cell lines derived from human somatic cells. Science 318, 1917–1920 (2007).
Hu, B.Y. & Zhang, S.C. Differentiation of spinal motor neurons from pluripotent human stem cells. Nat. Protoc. 4, 1295–1304 (2009).
Caldwell, M.A. et al. Growth factors regulate the survival and fate of cells derived from human neurospheres. Nat. Biotechnol. 19, 475–479 (2001).
Abe, K., Abe, Y. & Saito, H. Evaluation of L-glutamate clearance capacity of cultured rat cortical astrocytes. Biol. Pharm. Bull. 23, 204–207 (2000).
Acknowledgements
The authors thank A. Messing for critical reading of the manuscript. This study was supported by the ALS Association, National Institute of Neurological Disorders and Stroke (NS045926, NS057778, NS064578), National MS Society (NMSS TR-3761), NYSTEM (C024406), Bleser Family Foundation, Busta Family Foundation, Neuroscience Training Program (T32 GM007507) and partly by a core grant to the Waisman Center from the National Institute of Child Health and Human Development (P30 HD03352).
Author information
Authors and Affiliations
Contributions
R.K. and S.-C.Z. designed the experiments and wrote the manuscript. R.K., J.P.W., Y.L. and Z.-J.Z. performed the experiments. R.K., J.P.W., Y.L., Z.-.J.Z. and S.-C.Z. analyzed the data.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Tables 1 and 2 and Supplementary Figures 1–4 (PDF 802 kb)
Supplementary Movie 1
Representative example of calcium wave propagation in FGF8-specified astroglia. (AVI 2201 kb)
Supplementary Movie 2
Z series of human astrocyte image in Fig. 4g stained for GFAP (green), human nuclei (red), and total nuclei (blue). (AVI 612 kb)
Rights and permissions
About this article
Cite this article
Krencik, R., Weick, J., Liu, Y. et al. Specification of transplantable astroglial subtypes from human pluripotent stem cells. Nat Biotechnol 29, 528–534 (2011). https://doi.org/10.1038/nbt.1877
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nbt.1877
This article is cited by
-
Stem cell-derived brainstem mouse astrocytes obtain a neurotoxic phenotype in vitro upon neuroinflammation
Journal of Inflammation (2023)
-
The Ties That Bind: Glial Transplantation in White Matter Ischemia and Vascular Dementia
Neurotherapeutics (2023)
-
The Progress of Stem Cell Therapy in Myocardial-Infarcted Heart Regeneration: Cell Sheet Technology
Tissue Engineering and Regenerative Medicine (2022)
-
Downregulated Calcium-Binding Protein S100A16 and HSP27 in Placenta-Derived Multipotent Cells Induce Functional Astrocyte Differentiation
Stem Cell Reviews and Reports (2022)
-
Heterogeneity of white matter astrocytes in the human brain
Acta Neuropathologica (2022)