Abstract
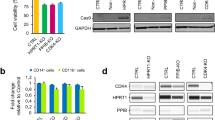
Human induced pluripotent stem cells (hiPSCs1,2,3) are useful in disease modeling and drug discovery, and they promise to provide a new generation of cell-based therapeutics. To date there has been no systematic evaluation of the most widely used techniques for generating integration-free hiPSCs. Here we compare Sendai-viral (SeV)4, episomal (Epi)5 and mRNA transfection mRNA6 methods using a number of criteria. All methods generated high-quality hiPSCs, but significant differences existed in aneuploidy rates, reprogramming efficiency, reliability and workload. We discuss the advantages and shortcomings of each approach, and present and review the results of a survey of a large number of human reprogramming laboratories on their independent experiences and preferences. Our analysis provides a valuable resource to inform the use of specific reprogramming methods for different laboratories and different applications, including clinical translation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Takahashi, K. et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131, 861–872 (2007).
Yu, J. et al. Induced pluripotent stem cell lines derived from human somatic cells. Science 318, 1917–1920 (2007).
Park, I.H. et al. Reprogramming of human somatic cells to pluripotency with defined factors. Nature 451, 141–146 (2008).
Fusaki, N. et al. Efficient induction of transgene-free human pluripotent stem cells using a vector based on Sendai virus, an RNA virus that does not integrate into the host genome. Proc. Jpn. Acad., Ser. B, Phys. Biol. Sci. 85, 348–362 (2009).
Okita, K. et al. A more efficient method to generate integration-free human iPS cells. Nat. Methods 8, 409–412 (2011).
Warren, L. et al. Highly efficient reprogramming to pluripotency and directed differentiation of human cells with synthetic modified mRNA. Cell Stem Cell 7, 618–630 (2010).
Yu, J. et al. Human induced pluripotent stem cells free of vector and transgene sequences. Science 324, 797–801 (2009).
Goh, P.A. et al. A systematic evaluation of integration free reprogramming methods for deriving clinically relevant patient specific induced pluripotent stem (iPS) cells. PLoS ONE 8, e81622 (2013).
Lieu, P.T. et al. Generation of induced pluripotent stem cells with CytoTune, a non-integrating Sendai virus. Methods Mol. Biol. 997, 45–56 (2013).
Warren, L., Ni, Y., Wang, J. & Guo, X. Feeder-free derivation of human induced pluripotent stem cells with messenger RNA. Sci. Rep. 2, 657 (2012).
Somers, A. Generation of transgene-free lung disease-specific human induced pluripotent stem cells using a single excisable lentiviral stem cell cassette. Stem Cells 28, 1728–1740 (2010).
Gore, A. et al. Somatic coding mutations in human induced pluripotent stem cells. Nature 471, 63–67 (2011).
Laurent, L.C. et al. Dynamic changes in the copy number of pluripotency and cell proliferation genes in human ESCs and iPSCs during reprogramming and time in culture. Cell Stem Cell 8, 106–118 (2011).
Lister, R. et al. Hotspots of aberrant epigenomic reprogramming in human induced pluripotent stem cells. Nature 471, 68–73 (2011).
Ruiz, S. et al. Identification of a specific reprogramming-associated epigenetic signature in human induced pluripotent stem cells. Proc. Natl. Acad. Sci. USA 109, 16196–16201 (2012).
Carey, B.W. et al. Reprogramming factor stoichiometry influences the epigenetic state and biological properties of induced pluripotent stem cells. Cell Stem Cell 9, 588–598 (2011).
Koyanagi-Aoi, M. et al. Differentiation-defective phenotypes revealed by large-scale analyses of human pluripotent stem cells. Proc. Natl. Acad. Sci. USA 110, 20569–20574 (2013).
Bock, C. et al. Reference maps of human ES and iPS cell variation enable high-throughput characterization of pluripotent cell lines. Cell 144, 439–452 (2011).
Buganim, Y. et al. The developmental potential of iPSCs is greatly influenced by reprogramming factor selection. Cell Stem Cell 15, 295–309 (2014).
Park, T.S. et al. Growth factor-activated stem cell circuits and stromal signals cooperatively accelerate non-integrated iPSC reprogramming of human myeloid progenitors. PLoS ONE 7, e42838 (2012).
Okita, K. et al. An efficient nonviral method to generate integration-free human-induced pluripotent stem cells from cord blood and peripheral blood cells. Stem Cells 31, 458–466 (2013).
Yu, J., Chau, K.F., Vodyanik, M.A., Jiang, J. & Jiang, Y. Efficient feeder-free episomal reprogramming with small molecules. PLoS ONE 6, e17557 (2011).
Chen, G. et al. Chemically defined conditions for human iPSC derivation and culture. Nat. Methods 8, 424–429 (2011).
Su, R.J. et al. Efficient generation of integration-free ips cells from human adult peripheral blood using BCL-XL together with Yamanaka factors. PLoS ONE 8, e64496 (2013).
Wang, Y. et al. Reprogramming of mouse and human somatic cells by high-performance engineered factors. EMBO Rep. 12, 373–378 (2011).
MacArthur, C.C. et al. Generation of human-induced pluripotent stem cells by a nonintegrating RNA Sendai virus vector in feeder-free or xeno-free conditions. Stem Cells Int. 2012, 564612 (2012).
Awe, J.P. et al. Generation and characterization of transgene-free human induced pluripotent stem cells and conversion to putative clinical-grade status. Stem Cell Res. Ther. 4, 87 (2013).
Nakagawa, M. et al. A novel efficient feeder-free culture system for the derivation of human induced pluripotent stem cells. Sci. Rep. 4, 3594 (2014).
Hou, P. et al. Pluripotent stem cells induced from mouse somatic cells by small-molecule compounds. Science 341, 651–654 (2013).
Jiang, J. et al. Zscan4 promotes genomic stability during reprogramming and dramatically improves the quality of iPS cells as demonstrated by tetraploid complementation. Cell Res. 23, 92–106 (2013).
Hartung, O., Huo, H., Daley, G.Q. & Schlaeger, T.M. Clump passaging and expansion of human embryonic and induced pluripotent stem cells on mouse embryonic fibroblast feeder cells. Curr. Protoc. Stem Cell Biol. 14, 1C.10 (2010).
Manos, P.D., Ratanasirintrawoot, S., Loewer, S., Daley, G.Q. & Schlaeger, T.M. Live-cell immunofluorescence staining of human pluripotent stem cells. Curr. Protoc. Stem Cell Biol. 19, 1C.12 (2011).
Somers, A. Generation of transgene-free lung disease-specific human induced pluripotent stem cells using a single excisable lentiviral stem cell cassette. Stem Cells 28, 1728–1740 (2010).
Park, I.H., Lerou, P.H., Zhao, R., Huo, H. & Daley, G.Q. Generation of human-induced pluripotent stem cells. Nat. Protoc. 3, 1180–1186 (2008).
Miller, J.D. & Schlaeger, T.M. Generation of induced pluripotent stem cell lines from human fibroblasts via retroviral gene transfer. Methods Mol. Biol. 767, 55–65 (2011).
Chan, E.M. et al. Live cell imaging distinguishes bona fide human iPS cells from partially reprogrammed cells. Nat. Biotechnol. 27, 1033–1037 (2009).
Bock, C. et al. Reference Maps of human ES and iPS cell variation enable high-throughput characterization of pluripotent cell lines. Cell 144, 439–452 (2011).
Hansen, K.D. et al. From whole genome bisulfite sequencing reads to differentially methylated regions. Genome Biol. 13, R83 (2012).
Hansen, K.D. et al. Increased methylation variation in epigenetic domains across cancer types. Nat. Genet. 43, 768–775 (2011).
Lister, R. et al. Hotspots of aberrant epigenomic reprogramming in human induced pluripotent stem cells. Nature 471, 68–73 (2011).
Acknowledgements
We would like to thank B. Hamilton (Stemgent) for miRNA reprogramming agents and protocols, G. Mostoslavsky (Boston University) for human STEMCCA lentiviral plasmid constructs, M. Armant (Boston Children's Hospital) for MRC5 and hCD34+ cells, G. MacLean (Boston Children's Hospital) for advice on episomal blood reprogramming and S. D'Souza (Icahn School of Medicine at Mount Sinai) for a list of contacts for human cell reprogramming laboratories. This work was supported in part by grants R01HL75737, U01HL107440, UO1-HL100001, U01HL87402 and U01HL100408 (National Heart, Lung, and Blood Institute (NHLBI) Progenitor Cell Biology Consortium), R24DK092760 (the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK)), PO1NS066888 (the National Institute of Neurological Disorders and Stroke (NINDS)), by the SMA Foundation, the Jerome Le Jeune Foundation, an EMBO postdoctoral fellowship (E.B.) and the Harvard Stem Cell Institute.
Author information
Authors and Affiliations
Contributions
T.M.S., L.D., C.A.C., A.M., L.L.R., A.P.F., L.I.Z. and G.Q.D. contributed to the conception and design of the study; T.M.S., L.D. and G.Q.D. wrote the manuscript; T.R.B., S.E., K.C., A.C., A.D., A.E., K.F., M.G., D.G., J.M., P.M., M.G. and B.B. performed reprogramming and cell culture experiments; A.D., N.J., X.L. and A.P.F. performed epigenetics analyses, T.M.S., K.F., D.G., P.M., L.D., T.R.B. and S.E. performed PCR assays; M.S.L., E.B., A.B.C.C. and D.D. provided cell samples and reprogramming data; A.M.T. and A.M. performed the scorecard analysis; T.M.S. performed statistical analyses; T.M.S. and L.D. performed the survey.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–7, Supplementary Table 1 and Supplementary Methods (PDF 3485 kb)
Rights and permissions
About this article
Cite this article
Schlaeger, T., Daheron, L., Brickler, T. et al. A comparison of non-integrating reprogramming methods. Nat Biotechnol 33, 58–63 (2015). https://doi.org/10.1038/nbt.3070
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nbt.3070
This article is cited by
-
Synthesis of colloidal silica nanofluid and assessment of its impact on interfacial tension (IFT) and wettability for enhanced oil recovery (EOR)
Scientific Reports (2024)
-
Integration-free induced pluripotent stem cells from three endangered Southeast Asian non-human primate species
Scientific Reports (2024)
-
WNT signalling control by KDM5C during development affects cognition
Nature (2024)
-
Epigenetic OCT4 regulatory network: stochastic analysis of cellular reprogramming
npj Systems Biology and Applications (2024)
-
Limitations of the human iPSC-derived neuron model for early-onset Alzheimer’s disease
Molecular Brain (2023)