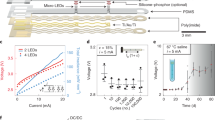
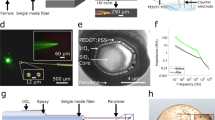
Abstract
Optogenetics allows rapid, temporally specific control of neuronal activity by targeted expression and activation of light-sensitive proteins. Implementation typically requires remote light sources and fiber-optic delivery schemes that impose considerable physical constraints on natural behaviors. In this report we bypass these limitations using technologies that combine thin, mechanically soft neural interfaces with fully implantable, stretchable wireless radio power and control systems. The resulting devices achieve optogenetic modulation of the spinal cord and peripheral nervous system. This is demonstrated with two form factors; stretchable film appliqués that interface directly with peripheral nerves, and flexible filaments that insert into the narrow confines of the spinal epidural space. These soft, thin devices are minimally invasive, and histological tests suggest they can be used in chronic studies. We demonstrate the power of this technology by modulating peripheral and spinal pain circuitry, providing evidence for the potential widespread use of these devices in research and future clinical applications of optogenetics outside the brain.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Iyer, S.M. et al. Virally mediated optogenetic excitation and inhibition of pain in freely moving nontransgenic mice. Nat. Biotechnol. 32, 274–278 (2014).
Towne, C., Montgomery, K.L., Iyer, S.M., Deisseroth, K. & Delp, S.L. Optogenetic control of targeted peripheral axons in freely moving animals. PLoS One 8, e72691 (2013).
Daou, I. et al. Remote optogenetic activation and sensitization of pain pathways in freely moving mice. J. Neurosci. 33, 18631–18640 (2013).
Kozai, T.D. et al. Ultrasmall implantable composite microelectrodes with bioactive surfaces for chronic neural interfaces. Nat. Mater. 11, 1065–1073 (2012).
Sparta, D.R. et al. Construction of implantable optical fibers for long-term optogenetic manipulation of neural circuits. Nat. Protoc. 7, 12–23 (2012).
Jang, K.I. et al. Rugged and breathable forms of stretchable electronics with adherent composite substrates for transcutaneous monitoring. Nat. Commun. 5, 4779 (2014).
Kim, D.H. et al. Epidermal electronics. Science 333, 838–843 (2011).
Kim, T.I. et al. Injectable, cellular-scale optoelectronics with applications for wireless optogenetics. Science 340, 211–216 (2013).
Xu, S. et al. Soft microfluidic assemblies of sensors, circuits, and radios for the skin. Science 344, 70–74 (2014).
Montgomery, K.L. et al. Wirelessly powered, fully internal optogenetics for brain, spinal and peripheral circuits in mice. Nat. Methods 12, 969–974 (2015).
Folcher, M. et al. Mind-controlled transgene expression by a wireless-powered optogenetic designer cell implant. Nat. Commun. 5, 5392 (2014).
Harrington, R.F. Time-Harmonic Electromagnetic Fields (Wiley-IEEE Press, 2001).
IEEE Standard for Safety Levels with Respect to Human Exposure to Radio Frequency Electromagnetic Fields, 3 kHz to 300 GHz, IEEE Standard C95.1-2005 (Institute of Electronic and Electrical Engineers, 2005).
da Silva, S. et al. Proper formation of whisker barrelettes requires periphery-derived Smad4-dependent TGF-beta signaling. Proc. Natl. Acad. Sci. USA 108, 3395–3400 (2011).
Hasegawa, H., Abbott, S., Han, B.X., Qi, Y. & Wang, F. Analyzing somatosensory axon projections with the sensory neuron-specific Advillin gene. J. Neurosci. 27, 14404–14414 (2007).
Fink, D.J. et al. Gene therapy for pain: results of a phase I clinical trial. Ann. Neurol. 70, 207–212 (2011).
Fink, D.J. & Wolfe, D. Gene therapy for pain: a perspective. Pain Manag. 1, 379–381 (2011).
Miyazato, M. et al. Suppression of detrusor-sphincter dyssynergia by herpes simplex virus vector mediated gene delivery of glutamic acid decarboxylase in spinal cord injured rats. J. Urol. 184, 1204–1210 (2010).
Yokoyama, H. et al. Gene therapy for bladder overactivity and nociception with herpes simplex virus vectors expressing preproenkephalin. Hum. Gene Ther. 20, 63–71 (2009).
Pleticha, J. et al. Preclinical toxicity evaluation of AAV for pain: evidence from human AAV studies and from the pharmacology of analgesic drugs. Mol. Pain 10, 54 (2014).
Agarwal, N., Offermanns, S. & Kuner, R. Conditional gene deletion in primary nociceptive neurons of trigeminal ganglia and dorsal root ganglia. Genesis 38, 122–129 (2004).
Mishra, S.K., Tisel, S.M., Orestes, P., Bhangoo, S.K. & Hoon, M.A. TRPV1-lineage neurons are required for thermal sensation. EMBO J. 30, 582–593 (2011).
Madisen, L. et al. A toolbox of Cre-dependent optogenetic transgenic mice for light-induced activation and silencing. Nat. Neurosci. 15, 793–802 (2012).
Bennett, G.J. & Xie, Y.K. A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain 33, 87–107 (1988).
Harrison, M. et al. Vertebral landmarks for the identification of spinal cord segments in the mouse. Neuroimage 68, 22–29 (2013).
Golden, J.P. et al. Dopamine-dependent compensation maintains motor behavior in mice with developmental ablation of dopaminergic neurons. J. Neurosci. 33, 17095–17107 (2013).
Montana, M.C. et al. The metabotropic glutamate receptor subtype 5 antagonist fenobam is analgesic and has improved in vivo selectivity compared with the prototypical antagonist 2-methyl-6-(phenylethynyl)-pyridine. J. Pharmacol. Exp. Ther. 330, 834–843 (2009).
Golden, J.P. et al. RET signaling is required for survival and normal function of nonpeptidergic nociceptors. J. Neurosci. 30, 3983–3994 (2010).
Acknowledgements
This work was supported by a US National Institutes of Health (NIH) Director's Transformative Research Award (NS081707) to R.W.G., J.A.R. and M.R.B. D.S.B. was supported by an NIH Ruth L. Kirschstein F31 Predoctoral Fellowship (1F31NS078852). C.D.M. was supported by a Howard Hughes Medical Institute (HHMI) Medical Research Fellowship. B.A.C. was supported by a W.M. Keck Fellowship in Molecular Medicine and TR32 GM108539. M.Y.P. was supported by T32 GM007067. S.D. was supported by NS076324. Illustrations created by J. Sinn-Hanlon and P. Focken, University of Illinois. The authors appreciate the gifts of heterozygous SNS-cre mice from R. Kuner (University of Heidelberg), heterozygous TrpV1-cre mice from M. Hoon (NIH/National Institute of Dental and Craniofacial Research) and heterozygous Advillin-cre mice from F. Wang (Duke University). We would also like to think R.E. Schmidt for the expertise he provided in neuropathological examination of tissue.
Author information
Authors and Affiliations
Contributions
S.I.P. designed wireless optoelectronic systems, fabricated devices, tested devices, made wireless measurements, conducted simulations of wireless performance, designed experiments, generated figures, wrote and edited the manuscript. D.S.B. designed sciatic nerve devices, implanted devices, tested mice behavior, designed experiments, performed immunostaining, generated figures, wrote and edited the manuscript. G.S. designed and fabricated spinal cord devices, tested devices, generated figures, wrote and edited the manuscript. C.D.M. designed spinal cord devices, implanted devices, tested mice in behavior, designed experiments, performed immunostaining, generated figures, wrote and edited the manuscript. B.A.C. performed immunostaining and quantification, electrophysiology experiments, generated figures. H.U.C. and K.N.N. fabricated devices and tested devices. M.Y.P. performed surgical procedures, behavioral studies and electrophysiology, generated figures and edited the manuscript. S.D. performed experiments, implanted devices, generated figures. S.J.O., J.Y. and K.-I.J. made contributions to fabrication and testing of devices. V.K.S. performed experiments, immunostaining and generated figures. M.N. performed immunostaining and quantification of slides, as well as mouse breeding. J.G.G.-R. performed experiments and generated figures. S.K.V. performed immunostaining and mouse breeding. S.S.S. performed immunostaining and mouse breeding. K.M.W. performed immunostaining. J.S.H. made contributions to fabrication and testing of devices. R.X., T.P. and Y.H. performed mechanical simulations of device tolerance levels. T.K. designed and tested wireless optoelectronic systems for sciatic nerve. M.C.M. designed experiments and generated figures. J.P.G. performed immunostaining, generated figures, performed behavioral experiments, helped develop epidural implants and edited the manuscript. M.R.B. designed experiments. R.W.G. and J.A.R. oversaw all experiments and data analysis, designed experiments and devices, wrote and edited the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Notes 1–8; Supplementary Figures 1–22; Supplementary Tables 1–2 (PDF 4753 kb)
Rights and permissions
About this article
Cite this article
Park, S., Brenner, D., Shin, G. et al. Soft, stretchable, fully implantable miniaturized optoelectronic systems for wireless optogenetics. Nat Biotechnol 33, 1280–1286 (2015). https://doi.org/10.1038/nbt.3415
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nbt.3415
This article is cited by
-
Multilayer stretchable electronics with designs enabling a compact lateral form
npj Flexible Electronics (2024)
-
Body-conformable light-emitting materials and devices
Nature Photonics (2024)
-
Wearable and Implantable Light-Emitting Diodes and Their Biomedical Applications
Korean Journal of Chemical Engineering (2024)
-
Fatigue-resistant hydrogel optical fibers enable peripheral nerve optogenetics during locomotion
Nature Methods (2023)
-
Flexible brain–computer interfaces
Nature Electronics (2023)