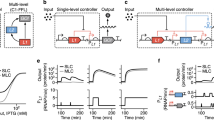
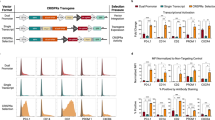
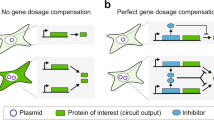
Abstract
The expression of transgenic proteins is often low and unstable over time, a problem that may be due to integration of the transgene in repressed chromatin. We developed a screening technology to identify genetic elements that efficiently counteract chromatin-associated repression. When these elements were used to flank a transgene, we observed a substantial increase in the number of mammalian cell colonies that expressed the transgenic protein. Expression of the shielded transgene was, in a copy number–dependent fashion, substantially higher than the expression of unprotected transgenes. Also, protein production remained stable over an extended time period. The DNA elements are small, not exceeding 2,100 base pairs (bp), and they are highly conserved between human and mouse, at both the functional and sequence levels. Our results demonstrate the existence of a class of genetic elements that can readily be applied to more efficient transgenic protein production in mammalian cells.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Change history
14 April 2003
This error has been corrected for the HTML (footnote, amended PDF until issue live) and the print versions of the article.
Notes
*Note: In the version of this article originally published online, the last two sentences of the first paragraph of Results are incorrect. The correct text should read: "Four independent screens were performed using either LexA-HPC2 or LexA-HP1 as repressor. This resulted in the recovery of 65 pSelect plasmids that conveyed survival (Fig. 1B)."
This error has been corrected for the HTML and the print versions of the article.
References
Gura, T. Magic bullets hit the target. Nature 417, 584–586 (2002).
Andersen, D.C. & Krummen, L. Recombinant protein expression for therapeutic applications. Curr. Opin. Biotechnol. 13, 117–123 (2002).
Pirrotta, V. PcG complexes and chromatin silencing. Curr. Opin. Genet. Dev. 7, 249–258 (1997).
Kingston, R.E., Bunker, C.A. & Imbalzano, A.N. Repression and activation by multiprotein complexes that alter chromatin structure. Genes Dev. 10, 905–920 (1996).
Satijn, D.P.E. et al. Interference with the expression of a novel human Polycomb protein, hPc2, results in cellular transformation and apoptosis. Mol. Cell. Biol. 17, 6076–6086 (1997).
Singh, P.B. et al. A sequence motif found in a Drosophila heterochromatin protein is conserved in animals and plants. Nucleic Acids Res. 19, 789–794 (1991).
Van der Vlag, J., Den Blaauwen, J., Sewalt, R.G.A.B., Van Driel, R., & Otte, A.P. Transcriptional repression mediated by Polycomb-group proteins and other chromatin-associated repressors is selectively blocked by boundary elements. J. Biol. Chem. 275, 697–704 (2000).
Zink, D. & Paro, R. Drosophila Polycomb group-regulated chromatin inhibits the accessibility of a trans-activator to its target DNA. EMBO J. 14, 5660–5671 (1995).
Ruezinsky, D., Beckman, H. & Kadesch, T. Modulation of the IgH enhancer's cell type specificity through a genetic switch. Genes Dev. 5, 29–37 (1991).
Cai, H. & Levine, M. Modulation of enhancer-promoter interactions by insulators in the Drosophila embryo. Nature 376, 533–536 (1995).
Chung, J.H., Whiteley, M. & Felsenfeld, G. A 5′ element of the chicken β-globin domain serves as an insulator in human erythroid cells and protects against position effect in Drosophila. Cell 74, 505–514 (1993).
Chung, J.H., Bell, A.C. & Felsenfeld, G. Characterization of the chicken β-globin insulator. Proc. Natl. Acad. Sci. USA 94, 575–580 (1997).
Van Blokland, R., Van der Geest, N., Mol, J.N.N. & Kooter, J.M. Transgene-mediated suppression of chalcone synthase expression in Petunia hybrida results from an increase in RNA turnover. Plant J. 6, 861–877 (1994).
Van der Vlag, J. & Otte, A.P. Transcriptional repression mediated by the human Polycomb-group protein EED involves histone deacetylation. Nat. Genet. 23, 474–478 (1999).
Lachner, M., O'Carroll, D., Rea, S., Mechtler, K. & Jenuwein, T. Methylation of histone H3 lysine 9 creates a binding site for HP1 proteins. Nature 410, 116–120 (2001).
Sewalt, R.G.A.B. et al. Selective interactions between vertebrate Polycomb homologs and the SUV39H1 HMTase suggest histone H3-K9 methylation to contribute to chromosomal targeting of Polycomb-group proteins. Mol. Cell. Biol. 22, 5539–5553 (2002).
Kuzmichev, A., Nishioka, K., Erdjument-Bromage, H., Tempst, P. & Reinberg, D. Histone methyltransferase activity associated with the human multiprotein complex containing Enhancer of Zeste protein. Genes Dev. 16, 2893–2905 (2002).
Ogawa, H., Ishiguro, K., Gaubatz, S., Livingston, D.M. & Nakatani, Y. A complex with chromatin modifiers that occupies E2F- and Myc-responsive genes in G0 cells. Science 296, 1132–1136 (2002).
Spellman, P.T. & Rubin, G.M. Evidence for large domains of similarly expressed genes in the Drosophila genome. J. Biol. 1, 5 (2002).
Bunker, C.A. & Kingston, R.E. Transcriptional repression by Drosophila and mammalian Polycomb group proteins in transfected mammalian cells. Mol. Cell. Biol. 14, 1721–1732 (1994).
Fu, P., Senior, P., Fernley, R.T., Tregear, G.W. & Aldred, G.P. Rapid determination of transgene copy number in stably-transfected mammalian cells by competitive PCR. J. Biochem. Biophys. Methods 40, 101–112 (1999).
Acknowledgements
We thank Valerie Dumay, Marike Feenstra, Thijs Hendrix, David Holmes, Mobien Kasiem, Ephie Kraneveld, Tim Segboer, and Johan van der Vlag for their involvement in the screens. P. Kwakman was sponsored by the Human Frontier Science Program (RG0039/1999-M).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
T.S., R.G.A.B.S, J.F.B., R.v.B., A.K., and A.P.O. are employed by ChromaGenics, a startup company that emerged from the Faculty of Sciences of the University of Amsterdam. ChromaGenics' mission is to create value through developing and commercializing proprietary technologies based on epigenetic gene regulation. ChromaGenics is within the compounds of the university and is financially supported by loans from the university and subsidies from the Ministry of Economical Affairs.
Supplementary information
Rights and permissions
About this article
Cite this article
Kwaks, T., Barnett, P., Hemrika, W. et al. Identification of anti-repressor elements that confer high and stable protein production in mammalian cells. Nat Biotechnol 21, 553–558 (2003). https://doi.org/10.1038/nbt814
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nbt814
This article is cited by
-
Optimization of piggyBac Transposon System Electrotransfection in Sheep Fibroblasts
Molecular Biotechnology (2023)
-
Chinese Hamster Ovary Cell Line Instability: Causes, Mitigation, and Prediction
Biotechnology and Bioprocess Engineering (2023)
-
A cell-based multiplex immunoassay platform using fluorescent protein-barcoded reporter cell lines
Communications Biology (2021)
-
Utilization of the human gamma-satellite insulator for the enhancement of anti-PCSK9 monoclonal antibody expression in Chinese hamster ovary cells
Molecular Biology Reports (2021)
-
Massively parallel RNA device engineering in mammalian cells with RNA-Seq
Nature Communications (2019)