Abstract
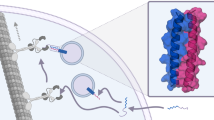
During the past fifteen years, a variety of peptides have been characterized for their ability to translocate into live cells. Most are efficient vectors that can internalize hydrophilic cargoes, and so provide a valuable biological (and potentially therapeutic) tool for targeting proteins into cells. Furthermore, translocation of cell-permeable peptides across the plasma membrane and their subsequent access to the cytosol, even when fused to large hydrophilic proteins, is challenging the perception of the plasma membrane as an impermeable barrier.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Frankel, A.D. & Pabo, C.O. Cellular uptake of the Tat protein from human immunodeficiency virus. Cell 55, 1189–1193 (1988).
Green, M. & Loewenstein, P.M. Autonomous functional domains of chemically synthesized human immunodeficiency virus tat trans-activator protein. Cell 55, 1179–1188 (1988).
Joliot, A., Pernelle, C., Deagostini-Bazin, H. & Prochiantz, A. Antennapedia homeobox peptide regulates neural morphogenesis. Proc. Natl Acad. Sci. USA 88, 1864–1868 (1991).
Chatelin, L., Volovitch, M., Joliot, A.H., Perez, F. & Prochiantz, A. Transcription factor Hoxa-5 is taken up by cells in culture and conveyed to their nuclei. Mech. Dev. 55, 111–117 (1996).
Helland, D.E., Welles, J.L., Caputo, A. & Haseltine, W.A. Transcellular transactivation by the human immunodeficiency virus type 1 tat protein. J. Virol. 65, 4547–4549 (1991).
Joliot, A. et al. Identification of a signal sequence necessary for the unconventional secretion of Engrailed homeoprotein. Curr. Biol. 8, 856–863 (1998).
Prochiantz, A. Messenger proteins: homeoproteins, TAT and others. Curr. Opin. Cell Biol. 12, 400–406 (2000).
Prochiantz, A. & Joliot, A. Can transcription factors function as cell–cell signalling molecules? Nature Rev. Mol. Cell. Biol. 4, 6–11 (2003).
Stenmark, H., Moskaug, J.O., Madshus, I.H., Sandvig, K. & Olsnes, S. Peptides fused to the amino-terminal end of diphteria toxin are translocated to the cytosol. J. Cell Biol. 113, 1025–1032 (1991).
Madshus, I., Stenmark, H., Sandvig, K. & Olsnes, S. Entry of Diphtheria toxin–Protein-A chimeras into cells. J. Biol. Chem. 266, 17446–17453 (1991).
Elliott, G. & O'Hare, P. Intercellular trafficking and protein delivery by a herpes virus structural protein. Cell 88, 223–233 (1997).
Lin, Y.Z., Yao, S.Y., Veach, R.A., Torgerson, T.R. & Hawiger, J. Inhibition of nuclear translocation of transcription factor NF-κB by a synthetic peptide containing a cell membrane-permeable motif and nuclear localization sequence. J. Biol. Chem. 270, 14255–14258 (1995).
Pooga, M., Hallbrink, M., Zorko, M. & Langel, U. Cell penetration by transportan. FASEB J. 12, 67–77 (1998).
Park, C.B., Yi, K.S., Matsuzaki, K., Kim, M.S. & Kim, S.C. Structure-activity analysis of buforin II, a histone H2A-derived antimicrobial peptide: the proline hinge is responsible for the cell-penetrating ability of buforin II. Proc. Natl Acad. Sci. USA 97, 8245–8250 (2000).
Derossi, D., Joliot, A.H., Chassaing, G. & Prochiantz, A. The third helix of Antennapedia homeodomain translocates through biological membranes. J. Biol. Chem. 269, 10444–10450 (1994).
Perez, F. et al. Antennapedia homeobox as a signal for the cellular internalization and nuclear addressing of a small exogenous peptide. J. Cell Sci. 102, 717–722 (1992).
Hall, H. et al. Inhibition of FGF-stimulated phosphatidylinositol hydrolysis and neurite outgrowth by a cell-membrane permeable phosphopeptide. Curr. Biol. 6, 580–587 (1996).
Pooga, M. et al. Cell penetrating PNA constructs regulate galanin receptor levels and modify pain transmission in vivo. Nature Biotechnol. 16, 857–861 (1998).
Schutze-Redelmeier, M.P. et al. Introduction of exogenous antigen into the MHC Class I processing and presentation pathway by Drosophila Antennapedia homeodomain primes cytotoxic T cells in vivo. J. Immunol. 157, 650–655 (1996).
Derossi, D. et al. Cell internalization of the third helix of the Antennapedia homeodomain is receptor-independent. J. Biol. Chem. 271, 18188–18193 (1996).
Jo, D. et al. Epigenetic regulation of gene structure and function with a cell-permeable Cre recombinase. Nature Biotechnol. 19, 929–933 (2001).
Peitz, M., Pfannkuche, K., Rajewsky, K. & Edenhofer, F. Ability of the hydrophobic FGF and basic TAT peptides to promote cellular uptake of recombinant Cre recombinase: a tool for efficient genetic engineering of mammalian genomes. Proc. Natl Acad. Sci. USA 99, 4489–4494 (2002).
Rousselle, C. et al. Enhanced delivery of doxorubicin into the brain via a peptide-vector-mediated strategy: saturation kinetics and specificity. J. Pharmacol. Exp. Ther. 296, 124–131 (2001).
Mazel, M. et al. Doxorubicin-peptide conjugates overcome multidrug resistance. Anticancer Drugs 12, 107–116 (2001).
Rousselle, C. et al. New advances in the transport of doxorubicin through the blood–brain barrier by a peptide vector-mediated strategy. Mol. Pharmacol. 57, 679–686 (2000).
Schwarze, S.R., Ho, A., Vocero-Akbani, A. & Dowdy, S.F. In vivo protein transduction: delivery of a biologically active protein into the mouse. Science. 285, 1569–1572 (1999).
Cao, G. et al. In vivo delivery of a Bcl-xL fusion protein containing the TAT protein transduction domain protects against ischemic brain injury and neuronal apoptosis. J. Neurosci. 22, 5423–5431 (2002).
Asoh, S. et al. Protection against ischemic brain injury by protein therapeutics. Proc. Natl Acad. Sci USA 99, 17107–17112 (2002).
Pietersz, G.A., Li, W. & Apostolopoulos, V. A 16-mer peptide (RQIKIWFQNRRMKWKK) from antennapedia preferentially targets the Class I pathway. Vaccine 19, 1397–1405 (2001).
Fahraeus, R., Paramio, J.M., Ball, K.L., Lain, S. & Lane, D.P. Inhibition of pRb phopshorylation and cell-cycle progresion by a 20-residue peptide derived from p16CDKN2/INK4A. Curr. Biol. 6, 84–91 (1996).
Herbert, T.P., Fahraeus, R., Prescott, A., Lane, D.P. & Proud, C.G. Rapid induction of apoptosis mediated by peptides that bind initiation factor eIF4E. Curr. Biol. 10, 793–796 (2000).
Mainguy, G. et al. An induction gene trap for identifying a homeoprotein-regulated locus. Nature Biotechnol. 18, 746–749 (2000).
Montesinos, M.L. et al. The neuronal microtubule-associated protein 1B is under homeoprotein transcriptional control. J. Neurosci. 21, 3350–3359 (2001).
Vives, E., Brodin, P. & Lebleu, B. A truncated HIV-1 Tat protein basic domain translocates through the plasma membrane and accumulates in the cell nucleus. J. Biol. Chem. 272, 16010–16017 (1997).
Ganz, T. Defensins: antimicrobial peptides of innate immunity. Nature Rev. Immunol. 3, 710–720 (2003).
Moskaug, J.O., Stenmark, H. & Olsnes, S. Insertion of diphteria toxin B-fragment into the plasma membrane at low pH. J. Biol. Chem. 266, 2652–2659 (1991).
Brasseur, R. Tilted peptides: a motif for membrane destabilization (hypothesis). Mol. Membr. Biol. 17, 31–40 (2000).
Berlose, J.P., Convert, O., Derossi, D., Brunissen, A. & Chassaing, G. Conformational and associative behaviors of the third helix of Antennapedia homeodomain in membrane-mimetic environments. Eur. J. Biochem. 242, 372–386 (1996).
Christiaens, B. et al. Tryptophan fluorescence study of the interaction of penetratin peptides with model membranes. Eur. J. Biochem. 269, 2918–2926 (2002).
Magzoub, M., Eriksson, L.E. & Graslund, A. Comparison of the interaction, positioning, structure induction and membrane perturbation of cell-penetrating peptides and non-translocating variants with phospholipid vesicles. Biophys Chem. 103, 271–288 (2003).
Dom, G. et al. Cellular uptake of Antennapedia Penetratin peptides is a two-step process in which phase transfer precedes a tryptophan-dependent translocation. Nucleic Acids Res. 31, 556–561 (2003).
Magzoub, M., Kilk, K., Eriksson, L.E., Langel, U. & Graslund, A. Interaction and structure induction of cell-penetrating peptides in the presence of phospholipid vesicles. Biochim. Biophys. Acta. 1512, 77–89 (2001).
Drin, G., Demene, H., Temsamani, J. & Brasseur, R. Translocation of the pAntp peptide and its amphipathic analogue AP-2AL. Biochemistry 40, 1824–1834 (2001).
Salamon, Z., Lindblom, G. & Tollin, G. Plasmon-waveguide resonance and impedance spectroscopy studies of the interaction between penetratin and supported lipid bilayer membranes. Biophys J. 84, 1796–1807 (2003).
Thoren, P.E., Persson, D., Karlsson, M. & Norden, B. The antennapedia peptide penetratin translocates across lipid bilayers — the first direct observation. FEBS Lett. 482, 265–268 (2000).
Drin, G. et al. Physico-chemical requirements for cellular uptake of pAntp peptide. Role of lipid-binding affinity. Eur. J. Biochem. 268, 1304–1314 (2001).
Oehlke, J. et al. Evidence for extensive and non-specific translocation of oligopeptides across plasma membranes of mammalian cells. Biochim. Biophys. Acta. 1330, 50–60 (1997).
Scheller, A., Wiesner, B., Melzig, M., Bienert, M. & Oehlke, J. Evidence for an amphipathicity independent cellular uptake of amphipathic cell-penetrating peptides. Eur. J. Biochem. 267, 6043–6050 (2000).
Wender, P.A. et al. The design, synthesis, and evaluation of molecules that enable or enhance cellular uptake: peptoid molecular transporters. Proc. Natl Acad. Sci. USA 97, 13003–13008 (2000).
Fischer, R., Waizenegger, T., Kohler, K. & Brock, R. A quantitative validation of fluorophore-labeled cell-permeable peptide conjugates: fluorophore and cargo dependence of import. Biochim. Biophys. Acta. 1564, 365–374 (2002).
Park, J. et al. Mutational analysis of a human immunodeficiency virus type 1 Tat protein transduction domain which is required for delivery of an exogenous protein into mammalian cells. J. Gen. Virol. 83, 1173–1181 (2002).
Sawada, M., Hayes, P. & Matsuyama, S. Cytoprotective membrane-permeable peptides designed from the Bax-binding domain of Ku70. Nature Cell Biol. 5, 352–357 (2003).
Bolton, S.J. et al. Cellular uptake and spread of the cell-permeable peptide penetratin in adult rat brain. Eur. J. Neurosci. 12, 2847–2855 (2000).
Joliot, A.H., Triller, A., Volovitch, M., Pernelle, C. & Prochiantz, A. α-2,8-Polysialic acid is the neuronal surface receptor of antennapedia homeobox peptide. New Biol. 3, 1121–1134 (1991).
Hong, F.D. & Clayman, G.L. Isolation of a peptide for targeted drug delivery into human head and neck solid tumors. Cancer Res. 60, 6551–6556 (2000).
Leifert, J.A., Holler, P.D., Harkins, S., Kranz, D.M. & Whitton, J.L. The cationic region from HIV tat enhances the cell-surface expression of epitope/MHC class I complexes. Gene Ther. 10, 2067–2073 (2003).
Lundberg, M. & Johansson, M. Positively charged DNA-binding proteins cause apparent cell membrane translocation. Biochem. Biophys. Res. Commun. 291, 367–371 (2002).
Kramer, S.D. & Wunderli-Allenspach, H. No entry for TAT(44–57) into liposomes and intact MDCK cells: novel approach to study membrane permeation of cell-penetrating peptides. Biochim. Biophys. Acta. 1609, 161–169 (2003).
Perez, F. et al. Rab3A and Rab3B carboxy-terminal peptides are potent and specific inhibitor of prolactin release by rat cultured anterior pituitary cells. Mol. Endocrinol. 8, 1278–1287 (1994).
Eguchi, A. et al. Protein transduction domain of HIV-1 Tat protein promotes efficient delivery of DNA into mammalian cells. J. Biol. Chem. 276, 26204–26210 (2001).
Futaki, S. et al. Arginine-rich peptides. An abundant source of membrane-permeable peptides having potential as carriers for intracellular protein delivery. J. Biol. Chem. 276, 5836–5840 (2001).
Gao, C. et al. A cell-penetrating peptide from a novel pVII-pIX phage-displayed random peptide library. Bioorg. Med. Chem. 10, 4057–4065 (2002).
Elmquist, A., Lindgren, M., Bartfai, T. & Langel, U. VE-cadherin-derived cell-penetrating peptide, pVEC, with carrier functions. Exp. Cell Res. 269, 237–244 (2001).
Lundberg, P. et al. Cell membrane translocation of the N-terminal (1–28) part of the prion protein. Biochem. Biophys. Res. Commun. 299, 85–90 (2002).
Klump, H. et al. Retroviral vector-mediated expression of HoxB4 in hematopoietic cells using a novel coexpression strategy. Gene Ther. 8, 811–817 (2001).
Horb, M.E., Shen, C.N., Tosh, D. & Slack, J.M. Experimental conversion of liver to pancreas. Curr. Biol. 13, 105–115 (2003).
Oehlke, J. et al. Cellular uptake of an α-helical amphipathic model peptide with the potential to deliver polar compounds into the cell interior non-endocytically. Biochim. Biophys. Acta. 1414, 127–139 (1998).
Morris, M.C., Depollier, J., Mery, J., Heitz, F. & Divita, G. A peptide carrier for the delivery of biologically active proteins into mammalian cells. Nature Biotechnol. 19, 1173–1176 (2001).
Acknowledgements
We thank M.-L. Montesinos, E. Dupont and S. Dupas for having provided some pictures from their work. The studies from our laboratories were supported by Centre National de la Recherche Scientifique, Ecole Normale Supérieure and grants from the Association Française de lutte contre les Myopathies (no. 7702), the Human Frontier Research Program (RGP26/2002) and the European Community (HPRN-CT-2001-00241).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Joliot, A., Prochiantz, A. Transduction peptides: from technology to physiology. Nat Cell Biol 6, 189–196 (2004). https://doi.org/10.1038/ncb0304-189
Issue Date:
DOI: https://doi.org/10.1038/ncb0304-189
This article is cited by
-
Inhibitory short peptides targeting EPS8/ABI1/SOS1 tri-complex suppress invasion and metastasis of ovarian cancer cells
BMC Cancer (2019)
-
Modifying PTEN recruitment promotes neuron survival, regeneration, and functional recovery after CNS injury
Cell Death & Disease (2019)
-
Modulation of UVB-induced Carcinogenesis by Activation of Alternative DNA Repair Pathways
Scientific Reports (2018)
-
Classification and function of small open reading frames
Nature Reviews Molecular Cell Biology (2017)
-
Effects of lactoferrin derived peptides on simulants of biological warfare agents
World Journal of Microbiology and Biotechnology (2017)