Abstract
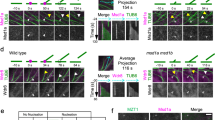
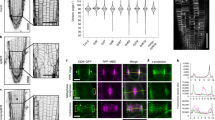
In plants, it is unclear how dispersed cortical microtubules are nucleated, polarized and organized in the absence of centrosomes. In Arabidopsis thaliana cells, expression of a fusion between the microtubule-end-binding protein AtEB1a and green fluorescent protein (GFP) results in labelling of spindle poles, where minus ends gather. During interphase, AtEB1a–GFP labels the microtubule plus end as a comet, but also marks the minus end as a site from which microtubules can grow and shrink. These minus-end nucleation sites are mobile, explaining how the cortical array can redistribute during the cell cycle and supporting the idea of a flexible centrosome in plants.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Lambert, A.-M. & Lloyd, C.W. in Microtubules (eds Hyams, J.S. & Lloyd, C.W.) 327–341 (Alan R Liss, New York, 1994).
Schmit, A.C. Acentrosomal microtubule nucleation in higher plants. Int. Rev. Cytol. 220, 257–289 (2002).
Yuan, M., Shaw, P.J., Warn, R.M. & Lloyd, C.W. Dynamic reorientation of cortical microtubules, from transverse to longitudinal, in living plant cells. Proc. Natl Acad. Sci. USA 91, 6050–6053 (1994).
Granger, C.L. & Cyr, R.J. Spatiotemporal relationships between growth and microtubule orientation as revealed in living root cells of Arabidopsis thaliana transformed with green-fluorescent-protein gene construct GFP–MBD. Protoplasma 216, 201–214 (2001).
Shaw, S.L., Kamyar, R. & Ehrhardt, D.W. Sustained microtubule treadmilling in Arabidopsis cortical arrays. Science 300, 1715–1718 (2003).
Mazia, D. Centrosomes and mitotic poles. Exp. Cell Res. 153, 1–15 (1984).
Drykova, D., Cenklova, V., Sulimenko, V., Draber, P. & Binarova, P. Plant γ-tubulin interacts with αβ dimers and form membrane-associated complexes. Plant Cell 15, 465–480 (2003).
Panteris, E., Apostolakos, P., Graf, R. & Galatis, B. γ-Tubulin colocalizes with microtubule arrays and tubulin paracrystals in dividing vegetative cells of higher plants. Protoplasma 210, 179–187 (2000).
Tirnauer, J.S. & Bierer, B.E. EB1 proteins regulate microtubule dynamics, cell polarity, and chromosome stability. J. Cell Biol. 149, 761–766 (2000).
Tirnauer, J.S., Grego, S., Salmon, E.D. & Mitchison, T.J. EB1–microtubule interactions in Xenopus egg extracts: role of EB1 in microtubule stabilization and mechanisms of targeting to microtubules. Mol. Biol. Cell 13, 3614–3626 (2002).
Mimori-Kiyosue, Y., Shiina, N. & Tsukita, S. The dynamic behavior of the APC-binding protein EB1 on the distal ends of microtubules. Curr. Biol. 10, 865–868 (2000).
Rogers, S.L., Rogers, G.C., Sharp, D.J. & Vale, R.D. Drosophila EB1 is important for proper assembly, dynamics, and positioning of the mitotic spindle. J. Cell Biol. 158, 873–884 (2002).
Korinek, W.S., Copeland, M.J., Chaudhuri, A. & Chant, J. Molecular linkage underlying microtubule orientation toward cortical sites in yeast. Science 287, 2257–2259 (2000).
Straube, A., Brill, M., Oakley, B.R., Horio, T. & Steinberg, G. Microtubule organization requires cell cycle-dependent nucleation at dispersed cytoplasmic sites: polar and perinuclear mcirotubule organizing centers in the plant pathogen Ustilago maydis. Mol. Biol. Cell 14, 642–657 (2003).
Morrison, E.E., Wardleworth, B.N., Askham, J.M., Markham, A.F. & Meredith, D.M. EB1, a protein which interacts with the APC tumour suppressor, is associated with the microtubule cytoskeleton throughout the cell cycle. Oncogene 17, 3471–3477 (1998).
Bu, W. & Su, L.K. Regulation of microtubule assembly by human EB1 family proteins. Oncogene 20, 3185–3192 (2001).
Morrison, E.E. & Ashkam, J.M. EB1 immunofluorescence reveals an increase in growing astral microtubule length and number during anaphase in NRK-52E cells. Eur. J. Cell Biol. 80, 749–753 (2001).
Rehberg, M. & Graf, R. Dictyostelium EB1 is a genuine centrosomal component required for proper spindle formation. Mol. Biol. Cell 13, 2301–2310 (2002).
Tirnauer, J.S., O'Toole, E., Berrueta, L., Bierer, B.E. & Pellman, D. Yeast Bim1p promotes the G1-specific dynamics of microtubules. J. Cell Biol. 145, 993–1007 (1999).
Askham, J.M., Vaughan, K.T., Goodson, H.V. & Morrison, E.E. Evidence that an interaction between EB1 and p150(Glued) is required for the formation and maintenance of a radial microtubule array anchored at the centrosome. Mol. Biol. Cell 13, 3627–3645 (2002).
Euteneuer, U. & McIntosh, J.R. Polarity of midbody and phragmoplast microtubules. Proc. Natl Acad. Sci. USA 78, 372–376 (1981).
Juwana, J.P. et al. EB/RP gene family encodes tubulin binding proteins. Int. J. Cancer 81, 275–284 (1999).
Dhonukshe, P. & Gadella, T.W. Alteration of microtubule dynamic instability during preprophase band formation revealed by yellow fluorescent protein-CLIP170 microtubule plus-end labeling. Plant Cell 15, 597–611 (2003).
Toso, R.J., Jordan, M.A., Farrell, K.W., Matsumoto, B. & Wilson, L. Kinetic stabilization of microtubule dynamic instability in vitro by vinblastine. Biochemistry 32, 1285–1293 (1993).
Yvon, A.M., Wadsworth, P. & Jordan, M.A. Taxol suppresses dynamics of individual microtubules in living human tumor cells. Mol. Biol. Cell 10, 947–959 (1999).
Rusan, N.M., Fagerstrom, C.J., Yvon, A.M. & Wadsworth, P. Cell cycle-dependent changes in microtubule dynamics in living cells expressing green fluorescent protein-α-tubulin. Mol. Biol. Cell 12, 971–980 (2001).
Muhua, L., Adames, N.R., Murphy, M.D., Shields, C.R. & Cooper, J.A. A cytokinesis checkpoint requiring the yeast homologue of an APC-binding protein. Nature 393, 487–491 (1998).
Wood, K.W., Sakowicz, R., Goldstein, L.S. & Cleveland, D.W. CENP-E is a plus end-directed kinetochore motor required for metaphase chromosome alignment. Cell 91, 357–366 (1997).
Rodionov, V., Nadezhdina, E. & Borisy, G. Centrosomal control of microtubule dynamics. Proc. Natl Acad. Sci. USA 96, 115–120 (1999).
Wasteneys, G.O. Microtubule organization in the green kingdom: chaos or self-order? J. Cell Sci. 115, 1345–1354 (2002).
Hong, B. et al. Identification of a calmodulin-regulated Ca++-ATPase in the endoplasmic reticulum. Plant Physiol. 119, 1165–1176 (1999).
Chan, J., Rutten, T. & Lloyd, C. Isolation of microtubule-associated proteins from carrot cytoskeletons: a 120 kDa MAP decorates all four microtubule arrays and the nucleus. Plant J. 10, 251–259 (1996).
Acknowledgements
This work was supported by a grant-in-aid from the Biotechnology and Biological Sciences Research Council to the John Innes Centre. We are grateful to B. Trevaskis of CSIRO Canberra for supplying the Gateway modified GFP vector. We thank P. Rossignol, O. Korolev and G. Roberts for assistance and M. Webb for reading the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Chan, J., Calder, G., Doonan, J. et al. EB1 reveals mobile microtubule nucleation sites in Arabidopsis. Nat Cell Biol 5, 967–971 (2003). https://doi.org/10.1038/ncb1057
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb1057
This article is cited by
-
Novel Arabidopsis microtubule-associated proteins track growing microtubule plus ends
BMC Plant Biology (2017)
-
Studies on suppressors of sav2/shade avoidance 2 revealed altered interaction at the interface of αβ-tubulin intradimer affects microtubule dynamics
Plant Growth Regulation (2017)
-
Inhibition of cell expansion by rapid ABP1-mediated auxin effect on microtubules
Nature (2014)
-
MAPs: cellular navigators for microtubule array orientations in Arabidopsis
Plant Cell Reports (2014)
-
Arabidopsis SABRE and CLASP interact to stabilize cell division plane orientation and planar polarity
Nature Communications (2013)