Abstract
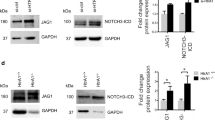
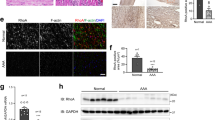
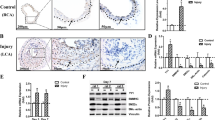
Vascular proliferative disorders, such as atherosclerosis and restenosis, are the most common causes of severe cardiovascular diseases, but a common molecular mechanism remains elusive. Here, we identify and characterize a novel hyperplasia suppressor gene, named HSG (later re-named rat mitofusin-2). HSG expression was markedly reduced in hyper-proliferative vascular smooth muscle cells (VSMCs) from spontaneously hypertensive rat arteries, balloon-injured Wistar Kyoto rat arteries, or ApoE-knockout mouse atherosclerotic arteries. Overexpression of HSG overtly suppressed serum-evoked VSMC proliferation in culture, and blocked balloon injury induced neointimal VSMC proliferation and restenosis in rat carotid arteries. The HSG anti-proliferative effect was mediated by inhibition of ERK/MAPK signalling and subsequent cell-cycle arrest. Deletion of the p21ras signature motif, but not the mitochondrial targeting domain, abolished HSG-induced growth arrest, indicating that rHSG-induced anti-proliferation was independent of mitochondrial fusion. Thus, rHSG functions as a cell proliferation suppressor, whereas dysregulation of rHSG results in proliferative disorders.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Novak, K. Cardiovascular disease increasing in developing countries. Nature Med. 4, 989–990 (1998).
Tunstall-Pedoe, H. et al. Contribution of trends in survival and coronary-event rates to changes in coronary heart disease mortality: 10-year results from 37 WHO MONICA project populations. Monitoring trends and determinants in cardiovascular disease. Lancet 353, 1547–1557 (1999).
Dzau, V.J., Braun-Dullaeus, R.C. & Sedding, D.G. Vascular proliferation and atherosclerosis: new perspectives and therapeutic strategies. Nature Med. 8, 1249–1256 (2002).
Forrester, J.S., Fishbein, M., Helfant, R. & Fagin, J. A paradigm for restenosis based on cell biology: clues for the development of new preventive therapies. J. Am. Coll. Cardiol. 17, 758–769 (1991).
Braun-Dullaeus, R.C., Mann, M.J. & Dzau, V.J. Cell cycle progression: new therapeutic target for vascular proliferative disease. Circulation 98, 82–89 (1998).
Libby, P. Vascular biology of atherosclerosis: overview and state of the art. Am. J. Cardiol. 91, 3A–6A (2003).
Nabel, E.G. et al. Recombinant platelet-derived growth factor B gene expression in porcine arteries induce intimal hyperplasia in vivo. J. Clin. Invest. 91, 1822–1829 (1993).
Nabel, E.G. et al. Recombinant fibroblast growth factor-1 promotes intimal hyperplasia and angiogenesis in arteries in vivo. Nature 362, 844–846 (1993).
Taylor, D.S. et al. Epiregulin is a potent vascular smooth muscle cell-derived mitogen induced by angiotensin II, endothelin-1, and thrombin. Proc. Natl Acad. Sci. 96, 1633–1638 (1999).
Dobrowolski, S., Harter, M. & Stacey, D.W. Cellular ras activity is required for passage through multiple points of the G0/G1 phase in BALB/c 3T3 cells. Mol. Cell Biol. 14, 5441–5449 (1994).
Marshall, C.J. Specificity of receptor tyrosine kinase signaling: transient versus sustained extracellular signal-regulated kinase activation. Cell 80, 179–185 (1995).
Peeper, D.S. et al. Ras signaling linked to the cell-cycle machinery by the retinoblastoma protein. Nature 386, 177–181 (1997).
Delmas, C. et al. The p42/p44 mitogen-activated protein kinase activation triggers p27kip1 degradation independently of cdk2/cyclin E in NIH 3T3 cells. J. Biol. Chem. 276, 34958–34965 (2001).
Leone, G., DeGregori, J., Sears, R., Jakoi, L. & Nevins, J.R. Myc and Ras collaborate in inducing accumulation of active cyclin E/Cdk2 and E2F. Nature 387, 422–426 (1997).
Aktas, H., Cai, H. & Cooper, G.M. Ras links growth factor signaling to the cell cycle machinery via regulation of cyclin D1 and the Cdk inhibitor p27KIP1. Mol. Cell. Biol. 17, 3850–3857 (1997).
Ferguson, J.E. & Patterson, C. Break the cycle: the role of cell-cycle modulation in the prevention of vasculoproliferative diseases. Cell Cycle 2, 211–219 (2003).
Santel, A. & Fuller, M.T. Control of mitochondrial morphology by a human mitofusin. J. Cell Sci. 114, 867–874 (2001).
Rojo, M., Legros, F., Chateau, D. & Lombès, A. Membrane topology and mitochondrial targeting of mitofusins, ubiquitous mammalian homologs of the transmembrane GTPase Fzo. J. Cell Sci. 115, 1663–1674 (2002).
Karbowski, M. et al. Spatial and temporal association of Bax with mitochondrial fission sites, Drp1, and Mfn2 during apoptosis. J. Cell Biol. 159, 931–938 (2002).
Chen, H. et al. Mitofusins Mfn1 and Mfn2 coordinately regulate mitochondrial fusion and are essential for embryonic development. J. Cell Biol. 160, 189–200 (2003).
Hales, K.G. & Fuller, M.T. Developmentally regulated mitochondrial fusion mediated by a conserved, novel, predicted GTPase. Cell 90, 121–129 (1997).
Okamoto, K. & Aoki, K. Development of a strain of spontaneously hypertensive rats. Jpn. Circ. J. 27, 282–293 (1963).
Johns, D.G., Webb, R.C. & Charpie, J.R. Impaired ceramide signaling in spontaneously hypertensive rat vascular smooth muscle: a possible mechanism for augmented cell proliferation. J. Hypertens. 19, 63–70 (2001).
Bing, O.H. et al. The spontaneously hypertensive rat as a model of the transition from compensated left ventricular hypertrophy to failure. J. Mol. Cell. Cardiol. 27, 383–396 (1995).
Clowes, A., Reidy, M. & Clowes, M. Kinetics of cellular proliferation after arterial injury. I. Smooth muscle growth in the absence of endothelium. Lab. Invest. 49, 327–333 (1983).
Clowes, A.W. & Schwartz, S.M. Significance of quiescent smooth muscle migration in the injured rat carotid artery. Circ. Res. 56, 139–145 (1985).
Hanke, H., Strohschneider, T., Oberhoff, M., Betz, E. & Karsch, K.R. Time course of smooth muscle cell proliferation in the intima and media of arteries following experimental angioplasty. Circ. Res. 67, 651–659 (1990).
Srivastava, S., Zou, Z.Q., Pirollo, K., Blattner, W. & Chang, E.H. Germ-line transmission of a mutated p53 gene in a cancer-prone family with Li-Fraumeni syndrome. Nature 348, 747–749 (1990).
Polyak, K. et al. Cloning of p27Kip1, a cyclin-dependent kinase inhibitor and a potential mediator of extracellular antimitogenic signals. Cell 78, 59–66 (1994).
Coats, S., Flanagan, W.M., Nourse, J. & Roberts, J.M. Requirement of p27Kip1 for restriction point control of the fibroblast cell cycle. Science 272, 877–880 (1996).
Nakayama, K. et al. Mice lacking p27Kip1 display increased body size, multiple organ hyperplasia, retinal dysplasia, and pituitary tumors. Cell 85, 707–720 (1996).
Fero, M.L. et al. A syndrome of multiorgan hyperplasia with features of gigantism, tumorigenesis, and female sterility in p27Kip1-deficient mice. Cell 85, 733–744 (1996).
Harbour, J.W. & Dean, D.C. Rb function in cell-cycle regulation and apoptosis. Nature Cell Biol. 2, E65–E67 (2000).
DeGregori, J., Kowalik, T. & Nevins, J.R. Cellular targets for activation by the E2F1 transcription factor include DNA synthesis- and G1/S-regulatory genes. Mol. Cell. Biol. 15, 4215–4224 (1995).
Garas, S.M., Huber, P. & Scott, N.A. Overview of therapies for prevention of restenosis after coronary interventions. Pharmacol. Ther. 92, 165–178 (2001).
Moses, J.W. et al. Sirolimus-eluting stents versus standard stents in patients with stenosis in a native coronary artery. N. Engl. J. Med. 349, 1315–1323 (2003).
Woods, T.C. & Marks, A.R. Drug eluting stents. Annu. Rev. Med. 55, 169–178 (2004).
Morishita, R. Recent progress in gene therapy for cardiovascular disease. Circ. J. 66, 1077–1086 (2002).
Chang, M.W. et al. Cytostatic gene therapy for vascular proliferative disorders with a constitutively active form of the retinoblastoma gene product. Science 267, 518–522 (1995).
Chang, M.W., Barr, E., Lu, M.M., Barton, K. & Leiden, J.M. Adenovirus-mediated over-expression of the cyclin/cyclin-dependent kinase inhibitor, p21 inhibits vascular smooth muscle cell proliferation and neointima formation in the rat carotid artery model of balloon angioplasty. J. Clin. Invest. 96, 2260–2268 (1995).
Ohno, T. et al. Gene therapy for vascular smooth muscle cell proliferation after arterial injury. Science 265, 781–784 (1994).
Ooboshi, H., Rios, C.D. & Heistad, D.D. Novel methods for adenovirus-mediated gene transfer to blood vessels in vivo. Mol. Cell. Biochem. 172, 37–46 (1997).
Tulis, D.A. et al. Adenovirus-mediated heme oxygenase-1 gene delivery inhibits injury-induced vascular neointima formation. Circulation 104, 2710–2715 (2001).
Maillard, L. et al. Effect of percutaneous adenovirus-mediated Gax gene delivery to the arterial wall in double-injured atheromatous stented rabbit iliac arteries. Gene Ther. 7, 1353–1361 (2000).
Kim, S. et al. Transcriptional targeting of replication-defective adenovirus transgene expression to smooth muscle cells in vivo. J. Clin. Invest. 100, 1006–1014 (1997).
Chakir, K. et al. The third intracellular loop and the carboxyl terminus of β2-adrenergic receptor confer the receptor spontaneous activity. Mol. Pharmacol. 64, 1048–1058 (2003).
Devlin, A.M. The effects of perindopril on vascular smooth muscle polyploidy in stroke-prone spontaneously hypertensive rats. J. Hypertens. 13, 211–218 (1995).
Feliciello, I & Chinali, G. A modified alkaline lysis method for the preparation of highly purified plasmid DNA from Escherichia coli. Anal. Biochem. 212, 394–401 (1993).
Benson, D.A. et al. GenBank. Nucleic Acids Res. 27, 12–17 (1999).
Acknowledgements
This work was supported by the National Basic Research Priorities Program 973 (G1998051015 and G2000056906), China's 863 High-Tech National Research Programme, Chinese Young Investigator Award (30225036), Peking University 985 Project. The authors would like to thank D. Longo, H. Cheng, E.G. Lakatta, P. Morin, X. Fu and J. Chen for critical reading and discussions. We would also like to thank M. Wang and J. Zhang for excellent technical support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information, Figures
Fig. S1, Fig. S2 and Fig. S3 (PDF 791 kb)
Rights and permissions
About this article
Cite this article
Chen, KH., Guo, X., Ma, D. et al. Dysregulation of HSG triggers vascular proliferative disorders. Nat Cell Biol 6, 872–883 (2004). https://doi.org/10.1038/ncb1161
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb1161
This article is cited by
-
A genetic mouse model of lean-NAFLD unveils sexual dimorphism in the liver-heart axis
Communications Biology (2024)
-
Small molecule agonist of mitochondrial fusion repairs mitochondrial dysfunction
Nature Chemical Biology (2023)
-
Integrated analysis of tRNA-derived small RNAs in proliferative human aortic smooth muscle cells
Cellular & Molecular Biology Letters (2022)
-
Cell death regulation by MAMs: from molecular mechanisms to therapeutic implications in cardiovascular diseases
Cell Death & Disease (2022)
-
MicroRNAs in hypertrophic cardiomyopathy: pathogenesis, diagnosis, treatment potential and roles as clinical biomarkers
Heart Failure Reviews (2022)