Abstract
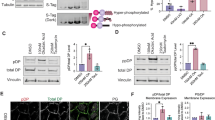
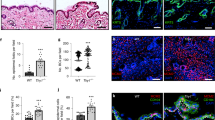
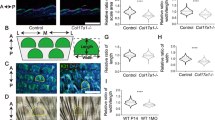
We have generated an epidermis-specific desmoplakin (DP) mouse knockout, and show that epidermal integrity requires DP; mechanical stresses to DP-null skin cause intercellular separations. The number of epidermal desmosomes in DP-null skin is similar to wild type (WT), but they lack keratin filaments, which compromise their function. DP-null keratinocytes have few desmosomes in vitro, and are unable to undergo actin reorganization and membrane sealing during epithelial sheet formation. Adherens junctions are also reduced. In vitro, DP transgene expression rescues these defects. DP is therefore required for assembly of functional desmosomes, maintaining cytoskeletal architecture and reinforcing membrane attachments essential for stable intercellular adhesion.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Garrod, D. R. Desmosomes and hemidesmosomes. Curr. Opin Cell. Biol. 5, 30–40 (1993).
Kowalczyk, A. P., Bornslaeger, E. A., Norvell, S. M., Palka, H. L. & Green, K. J. Desmosomes: intercellular adhesive junctions specialized for attachment of intermediate filaments. Int. Rev. Cytol. 185, 237–302 (1999).
Koch, P. J. & Franke, W. W. Desmosomal cadherins: another growing multigene family of adhesion molecules. Curr. Opin. Cell Biol. 6, 682–687 (1994).
Barth, A. I., Nathke, I. S. & Nelson, W. J. Cadherins, catenins and APC protein: interplay between cytoskeletal complexes and signaling pathways. Curr. Opin. Cell Biol. 9, 683–690 (1997).
Cowin, P., Kapprell, H.-P., Franke, W. W., Tamkun, J. & Hynes, R. O. Plakoglobin: a protein common to different kinds of intercellular adhering junctions. Cell 46, 1063–1073 (1986).
Mertens, C., Kuhn, C. & Franke, W. W. Plakophilins 2a and 2b: constitutive proteins of dual location in the karyoplasm and the desmosomal plaque. J. Cell Biol. 135, 1009–1025 (1996).
Schmidt, A. et al. Plakophilins 1a and 1b: widespread nuclear proteins recruited in specific epithelial cells as desmosomal plaque components. Cell Tissue Res. 290, 481–499 (1997).
Schmidt, A., et al. Plakophilin 3 — a novel cell-type-specific desmosomal plaque protein. Differentiation 64, 291–306 (1999).
Ruhrberg, C. & Watt, F. M. The plakin family: versatile organizers of cytoskeletal architecture. Curr. Opin. Genet. Dev. 7, 392–397 (1997).
Fuchs, E. & Karakesisoglou, I. Bridging cytoskeletal intersections. Genes Dev. 15, 1–14 (2001).
O'Keefe, E. J., Briggaman, R. A. & Herman, B. Calcium-induced assembly of adherens junctions in keratinocytes. J. Cell Biol. 105, 807–817 (1987).
Stappenbeck, T. S. & Green, K. J. The desmoplakin carboxyl terminus coaligns with and specifically disrupts intermediate filament networks when expressed in cultured cells. J. Cell Biol. 116, 1197–1209 (1992).
Kouklis, P., Hutton, E. & Fuchs, E. Making the connection: keratin intermediate filaments and desmosomes proteins. J. Cell Biol. 127, 1049–1060 (1994).
Smith, E. & Fuchs, E. Defining the interactions between intermediate filaments and desmosomes. J. Cell Biol. 141, 1229–1241 (1998).
Kowalczyk, A. P., et al. The amino-terminal domain of desmoplakin binds to plakoglobin and clusters desmosomal cadherin-plakoglobin complexes. J. Cell Biol. 139, 773–784 (1997).
Bornslaeger, E. A., Corcoran, C. M., Stappenbeck, T. S. & Green, K. J. Breaking the connection: displacement of the desmosomal plaque protein desmoplakin from cell-cell interfaces disrupts anchorage of intermediate filament bundles and alters intercellular junction assembly. J. Cell Biol. 134, 985–1001 (1996).
Gallicano, G. I., et al. Desmoplakin is required early in development for assembly of desmosomes and cytoskeletal linkage. J. Cell Biol. 143, 2009–2022 (1998).
Armstrong, D. K., et al. Haploinsufficiency of desmoplakin causes a striate subtype of palmoplantar keratoderma. Hum. Mol. Genet. 8, 143–148 (1999).
Norgett, E. E., et al. Recessive mutation in desmoplakin disrupts desmoplakin-intermediate filament interactions and causes dilated cardiomyopathy, woolly hair and keratoderma. Hum. Mol. Genet. 9, 2761–2766 (2000).
O'Gorman, S. & Wahl, G. M. Mouse engineering. Science 277, 1025 (1997).
Vasioukhin, V., Degenstein, L., Wise, B. & Fuchs, E. The magical touch: genome targeting in epidermal stem cells induced by tamoxifen application to mouse skin. Proc. Natl Acad. Sci. USA 96, 8551–8556 (1999).
Vasioukhin, V., Bauer, C., Yin, M. & Fuchs, E. Directed actin polymerization is the driving force for epithelial cell–cell adhesion. Cell 100, 209–219 (2000).
Rice, R. H. & Green, H. Presence in human epidermal cells of a soluble protein precursor of the cross-linked envelope: activation of the cross-linking by calcium ions. Cell 18, 681–694 (1979).
Nievers, M. G., Schaapveld, R. Q. & Sonnenberg, A. Biology and function of hemidesmosomes. Matrix Biol. 18, 5–17 (1999).
Jones, J. C., Hopkinson, S. B. & Goldfinger, L. E. Structure and assembly of hemidesmosomes. Bioessays 20, 488–494 (1998).
McGrath, J. A., et al. Mutations in the plakophilin 1 gene result in ectodermal dysplasia/skin fragility syndrome. Nature Genet. 17, 240–244 (1997).
Hatzfeld, M., Haffner, C., Schulze, K. & Vinzens, U. The function of plakophilin 1 in desmosome assembly and actin filament organization. J. Cell Biol. 149, 209–222 (2000).
Riddelle, K. S., Hopkinson, S. B. & Jones, J. C. Hemidesmosomes in the epithelial cell line 804G: their fate during wound closure, mitosis and drug induced reorganization of the cytoskeleton. J. Cell Sci. 103, 475–490 (1992).
Gumbiner, B., Stevenson, B. & Grimaldi, A. The role of the cell adhesion molecule uvomorulin in the formation and maintenance of the epithelial junctional complex. J. Cell Biol. 107, 1575–1587 (1988).
Lewis, J. E., Jensen, P. J. & Wheelock, M. J. Cadherin function is required for human keratinocytes to assemble desmosomes and stratify in response to calcium. J. Invest. Dermatol. 102, 870–877 (1994).
Wheelock, M. J. & Jensen, P. J. Regulation of keratinocyte intercellular junction organization and epidermal morphogenesis by E-cadherin. J. Cell Biol. 117, 415–425 (1992).
Lewis, J., et al. Cross-talk between adherens junctions and desmosomes depends on plakoglobin. J. Cell Biol. 136, 919–934 (1997).
Ruiz, P., et al. Targeted mutation of plakoglobin in mice reveals essential functions of desmosomes in the embryonic heart. J. Cell Biol. 135, 215–225 (1996).
Bierkamp, C., Mclaughlin, K. J., Schwarz, H., Huber, O. & Kemler, R. Embryonic heart and skin defects in mice lacking plakoglobin. Dev. Biol. 180, 780–785 (1996).
Andra, K., Nikolic, B., Stocher, M., Drenckhahn, D. & Wiche, G. Not just scaffolding: plectin regulates actin dynamics in cultured cells. Genes Dev. 12, 3442–3451 (1998).
Wang, X., Zinkel, S., Polansky, K. & Fuchs, E. Marked growth enhancement in transgenic mice expressing keratin promoter-driven human growth hormone: implications for keratinocyte mediated gene therapy. Proc. Natl Acad. Sci. USA 94, 219–226 (1997).
Haugwitz, M., Noegel, A. A., Karakesisoglou, I. & Schleicher, M. Dictyostelium amoebae that lack G-actin-sequestering profilins show defects in F-actin content, cytokinesis, and development. Cell 79, 303–314 (1994).
Acknowledgements
We thank I. King, J. Stanley; W. Franke for gifts of antibodies:. We especially thank A. Kobielak for carrying out the northern analysis. E.F. is an Investigator of the Howard Hughes Medical Institute. This work was supported by a grant to E.F. awarded by the National Institutes of Health (R01-AR27883).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Vasioukhin, V., Bowers, E., Bauer, C. et al. Desmoplakin is essential in epidermal sheet formation. Nat Cell Biol 3, 1076–1085 (2001). https://doi.org/10.1038/ncb1201-1076
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb1201-1076
This article is cited by
-
Photobiomodulation Facilitates Rat Cutaneous Wound Healing by Promoting Epidermal Stem Cells and Hair Follicle Stem Cells Proliferation
Tissue Engineering and Regenerative Medicine (2024)
-
Polarity signaling balances epithelial contractility and mechanical resistance
Scientific Reports (2023)
-
PP2A-B55alpha controls keratinocyte adhesion through dephosphorylation of the Desmoplakin C-terminus
Scientific Reports (2023)
-
Genetic lineage tracing identifies cardiac mesenchymal-to-adipose transition in an arrhythmogenic cardiomyopathy model
Science China Life Sciences (2023)
-
Distinct COPD subtypes in former smokers revealed by gene network perturbation analysis
Respiratory Research (2023)