Abstract
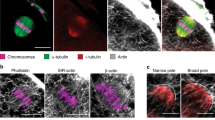
Actin is a major cytoskeletal element and is normally kept cytoplasmic by exportin 6 (Exp6)-driven nuclear export. Here, we show that Exp6 recognizes actin features that are conserved from yeast to human. Surprisingly however, microinjected actin was not exported from Xenopus laevis oocyte nuclei, unless Exp6 was co-injected, indicating that the pathway is inactive in this cell type. Indeed, Exp6 is undetectable in oocytes, but is synthesized from meiotic maturation onwards, which explains how actin export resumes later in embryogenesis. Exp6 thus represents the first example of a strictly developmentally regulated nuclear transport pathway. We asked why Xenopus oocytes lack Exp6 and observed that ectopic application of Exp6 renders the giant oocyte nuclei extremely fragile. This effect correlates with the selective disappearance of a sponge-like intranuclear scaffold of F-actin. These nuclei have a normal G2-phase DNA content in a volume 100,000 times larger than nuclei of somatic cells. Apparently, their mechanical integrity cannot be maintained by chromatin and the associated nuclear matrix, but instead requires an intranuclear actin-scaffold.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Accession codes
References
Fried, H. & Kutay, U. Nucleocytoplasmic transport: taking an inventory. Cell. Mol. Life Sci. 60, 1659–1688 (2003).
Görlich, D. & Kutay, U. Transport between the cell nucleus and the cytoplasm. Annu. Rev. Cell Dev. Biol. 15, 607–660 (1999).
Weis, K. Nucleocytoplasmic transport: cargo trafficking across the border. Curr. Opin. Cell Biol. 14, 328–335 (2002).
Ribbeck, K. & Görlich, D. Kinetic analysis of translocation through nuclear pore complexes. EMBO J. 20, 1320–1330 (2001).
Bohnsack, M. T. et al. Exp5 exports eEF1A via tRNA from nuclei and synergizes with other transport pathways to confine translation to the cytoplasm. EMBO J. 21, 6205–6215 (2002).
Stüven, T., Hartmann, E. & Görlich, D. Exportin 6: a novel nuclear export receptor that is specific for profilin.actin complexes. EMBO J. 22, 5928–5940 (2003).
Mingot, J. M. et al. Importin 13: a novel mediator of nuclear import and export. EMBO J. 20, 3685–3694 (2001).
Lipowsky, G. et al. Exportin 4: a mediator of a novel nuclear export pathway in higher eukaryotes. EMBO J. 19, 4362–4371 (2000).
Calado, A. et al. Exportin-5-mediated nuclear export of eukaryotic elongation factor 1A and tRNA. EMBO J. 21, 6216–6224 (2002).
Mingot, J., Bohnsack, M., Jäkle, U. & Görlich, D. Exportin 7/ RanBP16 defines a novel general nuclear export pathway. EMBO J. 24, 3227–3236 (2004).
Pollard, T. D. & Earnshaw, W. C. Cell Biology (W.B. Saunders, Philadelphia. 2002).
Clark, T. G. & Merriam, R. W. Diffusible and bound actin nuclei of Xenopus laevis oocytes. Cell 12, 883–891 (1977).
Bettinger, B. T., Gilbert, D. M. & Amberg, D. C. Actin up in the nucleus. Nature Rev. Mol. Cell Biol. 5, 410–415 (2004).
Pederson, T. & Aebi, U. Actin in the nucleus: what form and what for? J. Struct. Biol. 140, 3–9 (2002).
Hausen, P. & Riebesell, M. The Early Development of Xenopus laevis. (Springer, Berlin, Germany. 1991).
Nebreda, A. R. & Ferby, I. Regulation of the meiotic cell cycle in oocytes. Curr. Opin. Cell Biol. 12, 666–675 (2000).
Dancker, P., Low, I., Hasselbach, W. & Wieland, T. Interaction of actin with phalloidin: polymerization and stabilization of F-actin. Biochim. Biophys. Acta. 400, 407–414 (1975).
Jarmolowski, A., Boelens, W. C., Izaurralde, E. & Mattaj, I. W. Nuclear export of different classes of RNA is mediated by specific factors. J. Cell Biol. 124, 627–635 (1994).
Scheer, U., Hinssen, H., Franke, W. W. & Jockusch, B. M. Microinjection of actin-binding proteins and actin antibodies demonstrates involvement of nuclear actin in transcription of lampbrush chromosomes. Cell 39, 111–122 (1984).
Hu, P., Wu, S. & Hernandez, N. A role for b-actin in RNA polymerase III transcription. Genes Dev. 18, 3010–3015 (2004).
Hofmann, W. A. et al. Actin is part of pre-initiation complexes and is necessary for transcription by RNA polymerase II. Nature Cell Biol. 6, 1094–1101 (2004).
Görlich, D., Seewald, M. J. & Ribbeck, K. Characterization of Ran-driven cargo transport and the RanGTPase system by kinetic measurements and computer simulation. EMBO J. 22, 1088–1100 (2003).
Blessing, C. A., Ugrinova, G. T. & Goodson, H. V. Actin and ARPs: action in the nucleus. Trends Cell Biol. 14, 435–442 (2004).
Miralles, F., Posern, G., Zaromytidou, A. I. & Treisman, R. Actin dynamics control SRF activity by regulation of its coactivator MAL. Cell 113, 329–342 (2003).
Roeder, A. D. & Gard, D. L. Confocal microscopy of F-actin distribution in Xenopus oocytes. Zygote 2, 111–124 (1994).
Gard, D. L. Confocal microscopy and 3-D reconstruction of the cytoskeleton of Xenopus oocytes. Microsc. Res. Tech. 44, 388–414 (1999).
Gonsior, S. M. et al. Conformational difference between nuclear and cytoplasmic actin as detected by a monoclonal antibody. J. Cell Sci. 112, 797–809 (1999).
Cordes, V. C., Rackwitz, H. R. & Reidenbach, S. Mediators of nuclear protein import target karyophilic proteins to pore complexes of cytoplasmic annulate lamellae. Exp. Cell Res. 237, 419–433 (1997).
Kiseleva, E. et al. Actin- and protein-4.1-containing filaments link nuclear pore complexes to subnuclear organelles in Xenopus oocyte nuclei. J. Cell Sci. 117, 2481–2490 (2004).
Fukui, Y. & Katsumaru, H. Nuclear actin bundles in Amoeba, Dictyostelium and human HeLa cells induced by dimethyl sulfoxide. Exp. Cell Res. 120, 451–455 (1979).
Osborn, M. & Weber, K. Dimethylsulfoxide and the ionophore A23187 affect the arrangement of actin and induce nuclear actin paracrystals in PtK2 cells. Exp. Cell Res. 129, 103–114 (1980).
Sanger, J. W., Gwinn, J. & Sanger, J. M. Dissolution of cytoplasmic actin bundles and the induction of nuclear actin bundles by dimethyl sulfoxide. J. Exp. Zool. 213, 227–230 (1980).
Kwak, I. H. et al. Nuclear accumulation of globular actin as a cellular senescence marker. Cancer Res. 64, 572–580 (2004).
Iida, K., Iida, H. & Yahara, I. Heat shock induction of intranuclear actin rods in cultured mammalian cells. Exp. Cell Res. 165, 207–215 (1986).
Thiebaud, C. H. & Fischberg, M. DNA content in the genus Xenopus. Chromosoma 59, 253–257 (1977).
De Robertis, E. M., Longthorne, R. F. & Gurdon, J. B. Intracellular migration of nuclear proteins in Xenopus oocytes. Nature 272, 254–256 (1978).
Dingwall, C., Sharnick, S. V. & Laskey, R. A. A polypeptide domain that specifies migration of nucleoplasmin into the nucleus. Cell 30, 449–458 (1982).
Handwerger, K. E., Cordero, J. A. & Gall, J. G. Cajal bodies, nucleoli, and speckles in the Xenopus oocyte nucleus have a low-density, sponge-like structure. Mol. Biol. Cell 16, 202–211 (2005).
Dahl, K. N., Kahn, S. M., Wilson, K. L. & Discher, D. E. The nuclear envelope lamina network has elasticity and a compressibility limit suggestive of a molecular shock absorber. J. Cell Sci. 117, 4779–4786 (2004).
Boal, D. Mechanics of the Cell, (Cambridge University Press, Cambridge, UK. 2002).
Aebi, U., Millonig, R., Salvo, H. & Engel, A. The three-dimensional structure of the actin filament revisited. Ann. NY Acad. Sci. 483, 100–119 (1986).
Kaffman, A. & O'Shea, E. K. Regulation of nuclear localization: a key to a door. Annu. Rev. Cell Dev. Biol. 15, 291–339 (1999).
Ribbeck, K. & Görlich, D. The permeability barrier of nuclear pore complexes appears to operate via hydrophobic exclusion. EMBO J. 21, 2664–2671 (2002).
Acknowledgements
We wish to thank P. Rübmann, U. Jäkle and the animal facility of the ZMBH for excellent technical help, and J. Gall as well as the members of our laboratory for very helpful comments on the manuscript. This work received financial support from the Deutsche Forschungsgemeinschaft (SFB 638) and the Alfried–Krupp Foundation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Movie S1 (MOV 377 kb)
Supplementary Information
Supplementary Movie S2 (MOV 289 kb)
Rights and permissions
About this article
Cite this article
Bohnsack, M., Stüven, T., Kuhn, C. et al. A selective block of nuclear actin export stabilizes the giant nuclei of Xenopus oocytes. Nat Cell Biol 8, 257–263 (2006). https://doi.org/10.1038/ncb1357
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb1357
This article is cited by
-
Nuclear smooth muscle α-actin participates in vascular smooth muscle cell differentiation
Nature Cardiovascular Research (2023)
-
Nuclear actin filaments in DNA repair dynamics
Nature Cell Biology (2019)
-
Actin, actin-binding proteins, and actin-related proteins in the nucleus
Histochemistry and Cell Biology (2016)
-
Soft viscoelastic properties of nuclear actin age oocytes due to gravitational creep
Scientific Reports (2015)
-
β- and γ-Actins in the nucleus of human melanoma A375 cells
Histochemistry and Cell Biology (2015)