Abstract
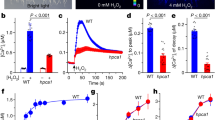
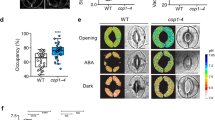
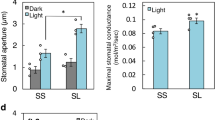
Guard cells, which form stomata in leaf epidermes, sense a multitude of environmental signals and integrate this information to regulate stomatal movements1,2. Compared with the advanced understanding of light and water stress responses in guard cells2,3,4, the molecular mechanisms that underlie stomatal CO2 signalling have remained relatively obscure. With a high-throughput leaf thermal imaging CO2 screen, we report the isolation of two allelic Arabidopsis mutants (high leaf temperature 1; ht1-1 and ht1-2) that are altered in their ability to control stomatal movements in response to CO2. The strong allele, ht1-2, exhibits a markedly impaired CO2 response but shows functional responses to blue light, fusicoccin and abscisic acid (ABA), indicating a role for HT1 in stomatal CO2 signalling. HT1 encodes a protein kinase that is expressed mainly in guard cells. Phosphorylation assays demonstrate that the activity of the HT1 protein carrying the ht1-1 or ht1-2 mutation is greatly impaired or abolished, respectively. Furthermore, dominant-negative HT1(K113W) transgenic plants, which lack HT1 kinase activity, show a disrupted CO2 response. These findings indicate that the HT1 kinase is important for regulation of stomatal movements and its function is more pronounced in response to CO2 than it is to ABA or light.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Accession codes
References
Willmer, C. M. & Fricker, M. D. Stomata 2nd edn (Chapman & Hall, London, 1996).
MacRobbie, E. A. C. Signal transduction and ion channels in guard cells. Phil. Trans. R. Soc. Lond. B. 353, 1475–1488 (1998).
Schroeder, J. I., Kwak, J. M. & Allen, G. J. Guard cell abscisic acid signalling and engineering drought hardiness in plants. Nature 410, 327–330 (2001).
Kinoshita, T et al. Phot1 and phot2 mediate blue light regulation of stomatal opening. Nature 414, 656–660 (2001).
Morison, J. I. L. in: Stomatal Function (eds Zeiger, E., Farquhar, G. D. and Cowan, I. R.) 229–251 (Stanford Univ. Press, California, 1987).
Legget, J., Pepper, W. J. & Swart, R. J. Climate Change 1992: The Supplementary Report to the IPCC Scientific Assessment 69–95 (Cambridge Univ. Press, Cambridge, UK, 1992).
Medlyn, B. E. et al. Stomatal conductance of forest species after long-term exposure to elevated CO2 concentration: a synthesis. New Phytol. 149, 247–264 (2001).
Schroeder, J. I., Allen, G. J., Hugouvieux, V., Kwak, J. M. & Waner, D. Guard cell signal transduction. Annu. Rev. Plant Physiol. Plant Mol. Biol. 52, 627–658 (2001).
Vavasseur, A. & Raghavendra, A. S. Guard cell metabolism and CO2 sensing. New Phytol. 165, 665–682 (2005).
Webb, A. A. R., McAinsh, M. R., Mansfield, T. A. & Hetherington, A. M. Carbon dioxide induces increases in guard cell cytosolic free calcium. Plant J. 9, 297–304 (1996).
Schwartz, A., Ilan, N. & Grantz, D. A. Calcium effects on stomatal movement in Commelina communis L. — use of EGTA to modulate stomatal response to light, KCl and CO2 . Plant Physiol. 87, 583–587 (1988).
Brearley, J., Venis, M. A. & Blatt, M. R. The effect of elevated CO2 concentrations on K+ and anion channels of Vicia faba L. guard cells. Planta 203, 145–154 (1997).
Assmann, S. M. The cellular basis of guard cell sensing of rising CO2 . Plant Cell Environ. 22, 629–637 (1999).
Hanstein, S. M. & Felle, H. H. CO2-triggered chloride release from guard cells in intact fava bean leaves. Kinetics of the onset of stomatal closure. Plant Physiol. 130, 940–950 (2002).
Raschke, K., Shabahang, M. & Wolf, R. The slow and the quick anion conductance in whole guard cells: their voltage-dependent alternation, and the modulation of their activities by abscisic acid and CO2 . Planta 217, 639–650 (2003).
Merlot, S. et al. Use of infrared thermal imaging to isolate Arabidopsis mutants defective in stomatal regulation. Plant J. 30, 601–609 (2002).
Kinoshita, T. & Shimazaki, K. Analysis of the phosphorylation level in guard-cell plasma membrane H+-ATPase in response to fusicoccin. Plant Cell Physiol. 42, 424–432 (2001).
Olsen, R. L., Pratt, R. B., Gump, P., Kemper, A. & Tallman, G. Red light activates a chloroplast-dependent ion uptake mechanism for stomatal opening under reduced CO2 concentrations in Vicia spp. New Phytol. 153, 497–508 (2002).
Roelfsema, M. R. G., Hanstein, S., Felle, H. H. & Hedrich, R. CO2 provides an intermediate link in the red light response of guard cells. Plant J. 32, 65–75 (2002).
Cutler, S., Ghassemian, M., Bonetta, D., Cooney, S. & McCourt, P. A protein farnesyl transferase involved in abscisic acid signal transduction in Arabidopsis. Science 273, 1239–1241 (1996).
Pei, Z.-M., Ghassemian, M., Kwak, C. M., McCourt, P. & Schroeder, J. I. Role of farnesyltransferase in ABA regulation of guard cell anion channels and plant water loss. Science 282, 287–290 (1998).
Leonhardt, N. et al. Microarray expression analyses of Arabidopsis guard cells and isolation of a recessive abscisic acid hypersensitive protein phosphatase 2C mutant. Plant Cell 16, 596–615 (2004).
Hanks, S. K., Quinn, A. M. & Hunter, T. The protein kinase family: Conserved features and deduced phylogeny of the catalytic domains. Science 241, 42–52 (1988).
Soyano, T., Nishihama, R., Morikiyo, K., Ishikawa, M. & Machida, Y. NQK1/NtMEK1 is a MAPKK that acts in the NPK1 MAPKKK-mediated MAPK cascade and is required for plant cytokinesis. Genes Dev. 17, 1055–1067 (2003).
Takekawa, M. & Saito, H. A family of stress-inducible GADD45-like proteins mediate activation of the stress-responsive MTK1/MEKK4 MAPKKK. Cell 95, 521–530 (1998).
Li, J., Wang, X.-Q., Watson, M. B. & Assmann, S. M. Regulation of abscisic acid-induced stomatal closure and anion channels by guard cell AAPK kinase. Science 287, 300–303 (2000).
Kieber, J. J., Rothenberg, M., Roman, G., Feldmann, K. A. & Ecker, J. R. CTR1, a negative regulator of the ethylene response pathway in Arabidopsis, encodes a member of the Raf family of protein kinases. Cell 72, 427–441 (1993).
Hua, J. & Meyerowitz, E. M. Ethylene responses are negatively regulated by a receptor gene family in Arabidopsis thaliana. Cell 94, 261–271 (1998).
Hetherington, A. M. & Woodward, F. I. The role of stomata in sensing and driving environmental change. Nature 424, 901–908 (2003).
Guo, F.-Q., Young, J. & Crawford, N. M. The nitrate transporter AtNRT1.1 (CHL1) functions in stomatal opening and contributes to drought susceptibility in Arabidopsis. Plant Cell 15, 107–117 (2003).
Pei, Z.-M., Kuchitsu, K., Ward, J. M., Schwarz, M. & Schroeder, J. I. Differential abscisic acid regulation of guard cell slow anion channels in Arabidopsis wild-type and abi1 and abi2 mutants. Plant Cell 9, 409–423 (1997).
Sugimoto, H. et al. The virescent-2 mutation inhibits translation of plastid transcripts for the plastid genetic system at an early stage of chloroplast differentiation. Plant Cell Physiol. 45, 985–996 (2004).
Mustilli, A.-C., Merlot, S., Vavasseur, A., Fenzi, F. & Giraudat, J. Arabidopsis OST1 protein kinase mediates the regulation of stomatal aperture by abscisic acid and acts upstream of reactive oxygen species production. Plant Cell 14, 3089–3099 (2002).
Acknowledgements
We thank Y. Machida and K. Harada, K.M. Kawano, E. Kasuya and all of the members of our laboratories for technical assistance and discussion. We also thank the Arabidopsis Biological Resource Center and Cereon Genomics for access to polymorphism information. This research was supported by CREST, JST and the Japan Society of the Promotion of Science (17370019) grants (K.I.), and by National Science Foundation (MCB0417118) and the National Institutes of Health (R01GM060396) grants (J.I.S.).M.I. is a Formas post-doctoral fellow.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Figure S1 (PDF 300 kb)
Rights and permissions
About this article
Cite this article
Hashimoto, M., Negi, J., Young, J. et al. Arabidopsis HT1 kinase controls stomatal movements in response to CO2. Nat Cell Biol 8, 391–397 (2006). https://doi.org/10.1038/ncb1387
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb1387
This article is cited by
-
Does MPK4/12-HT1 function as a CO2/bicarbonate sensor to regulate the stomatal conductance under high CO2 levels?
Plant Cell Reports (2023)
-
A pyrenoid-localized protein SAGA1 is necessary for Ca2+-binding protein CAS-dependent expression of nuclear genes encoding inorganic carbon transporters in Chlamydomonas reinhardtii
Photosynthesis Research (2023)
-
Arabidopsis guard cell CO2/HCO3− response mutant screening by an aequorin-based calcium imaging system
Plant Methods (2020)
-
Hoechst-tagged Fluorescein Diacetate for the Fluorescence Imaging-based Assessment of Stomatal Dynamics in Arabidopsis thaliana
Scientific Reports (2020)
-
Multispecies genome-wide analysis defines the MAP3K gene family in Gossypium hirsutum and reveals conserved family expansions
BMC Bioinformatics (2019)