Abstract
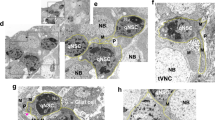
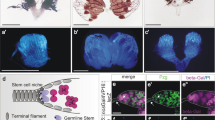
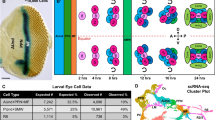
Stem cells generate self-renewing and differentiating progeny over many rounds of asymmetric divisions. How stem cell growth rate and size are maintained over time remains unknown. We isolated mutations in a Drosophila melanogaster gene, wicked (wcd), which induce premature differentiation of germline stem cells (GSCs). Wcd is a member of the U3 snoRNP complex required for pre-ribosomal RNA maturation. This general function of Wcd contrasts with its specific requirement for GSC self-renewal. However, live imaging of GSCs within their niche revealed a pool of Wcd-forming particles that segregate asymmetrically into the GSCs on mitosis, independently of the Dpp signal sent by the niche. A fraction of Wcd also segregated asymmetrically in dividing larval neural stem cells (NSCs). In the absence of Wcd, NSCs became smaller and produced fewer neurons. Our results show that regulation of ribosome synthesis is a crucial parameter for stem cell maintenance and function.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Birnbaum, K. D. & Sanchez Alvarado, A. Slicing across kingdoms: regeneration in plants and animals. Cell 132, 697–710 (2008).
Morrison, S. J. & Spradling, A. C. Stem cells and niches: mechanisms that promote stem cell maintenance throughout life. Cell 132, 598–611 (2008).
Gilboa, L. & Lehmann, R. How different is Venus from Mars? The genetics of germ-line stem cells in Drosophila females and males. Development 131, 4895–4905 (2004).
Wong, M. D., Jin, Z. & Xie, T. Molecular mechanisms of germline stem cell regulation. Annu. Rev. Genet. 39, 173–195 (2005).
Song, X. & Xie, T. DE-cadherin-mediated cell adhesion is essential for maintaining somatic stem cells in the Drosophila ovary. Proc. Natl Acad. Sci. USA 99, 14813–14818 (2002).
Xie, T. & Spradling, A. C. Decapentaplegic is essential for the maintenance and division of germline stem cells in the Drosophila ovary. Cell 94, 251–260 (1998).
Fichelson, P. & Huynh, J. R. Asymmetric divisions of germline cells. Prog. Mol. Subcell. Biol. 45, 97–120 (2007).
Deng, W. & Lin, H. Spectrosomes and fusomes anchor mitotic spindles during asymmetric germ cell divisions and facilitate the formation of a polarized microtubule array for oocyte specification in Drosophila. Dev. Biol. 189, 79–94 (1997).
Xie, T. & A, S. A niche maintaining germline stem cells in the Drosophila ovary. Science 290, 328–330 (2000).
de Cuevas, M., Lilly, M. A. & Spradling, A. C. Germline cyst formation in Drosophila. Annu. Rev. Genet. 31, 405–428 (1997).
Huynh, J. R. & St Johnston, D. The origin of asymmetry: early polarisation of the Drosophila germline cyst and oocyte. Curr. Biol. 14, R438–449 (2004).
de Cuevas, M. & Spradling, A. C. Morphogenesis of the Drosophila fusome and its implications for oocyte specification. Development 125, 2781–2789 (1998).
Huynh, J. R. Fusome as a cell–cell communication channel of Drosophila ovarian cyst (Landes Biosciences, 2006).
Neumuller, R. A. et al. Mei-P26 regulates microRNAs and cell growth in the Drosophila ovarian stem cell lineage. Nature (2008).
Rudra, D. & Warner, J. R. What better measure than ribosome synthesis? Genes Dev. 18, 2431–2436 (2004).
Frescas, D., Guardavaccaro, D., Bassermann, F., Koyama-Nasu, R. & Pagano, M. JHDM1B/FBXL10 is a nucleolar protein that represses transcription of ribosomal RNA genes. Nature 450, 309–313 (2007).
Maaloe, O. & Kjedgaard, N. Control of macromolecular synthesis, (W. A. Benjamin, New York, 1966).
Boisvert, F. M., van Koningsbruggen, S., Navascues, J. & Lamond, A. I. The multifunctional nucleolus. Nature Rev. Mol. Cell Biol. 8, 574–585 (2007).
Matera, A. G., Terns, R. M. & Terns, M. P. Non-coding RNAs: lessons from the small nuclear and small nucleolar RNAs. Nature Rev. Mol. Cell Biol. 8, 209–220 (2007).
Xu, T. & Rubin, G. Analysis of genetic mosaics in developing an adult Drosphila tissues. Development 117, 1223–1237 (1993).
Scherl, A. et al. Functional proteomic analysis of human nucleolus. Mol. Biol. Cell 13, 4100–4109 (2002).
Dragon, F. et al. A large nucleolar U3 ribonucleoprotein required for 18S ribosomal RNA biogenesis. Nature 417, 967–970 (2002).
Godfrey, A. C. et al. U7 snRNA mutations in Drosophila block histone pre-mRNA processing and disrupt oogenesis. RNA 12, 396–409 (2006).
Lanzotti, D. J. et al. Drosophila stem-loop binding protein intracellular localization is mediated by phosphorylation and is required for cell cycle-regulated histone mRNA expression. Mol. Biol. Cell 15, 1112–1123 (2004).
Liu, J. L. et al. The Drosophila melanogaster Cajal body. J. Cell Biol. 172, 875–884 (2006).
Long, E. O. & Dawid, I. B. Alternative pathways in the processing of ribosomal RNA precursor in Drosophila melanogaster. J. Mol. Biol. 138, 873–878 (1980).
Giordano, E., Peluso, I., Senger, S. & Furia, M. minifly, a Drosophila gene required for ribosome biogenesis. J. Cell Biol. 144, 1123–1133 (1999).
Chen, D. & McKearin, D. Dpp signaling silences bam transcription directly to establish asymmetric divisions of germline stem cells. Curr. Biol. 13, 1786–1791 (2003).
Casanueva, M. O. & Ferguson, E. L. Germline stem cell number in the Drosophila ovary is regulated by redundant mechanisms that control Dpp signaling. Development 131, 1881–1890 (2004).
Song, X. et al. Bmp signals from niche cells directly repress transcription of a differentiation-promoting gene, bag of marbles, in germline stem cells in the Drosophila ovary. Development 131, 1353–1364 (2004).
Bolivar, J., Pearson, J., Lopez-Onieva, L. & Gonzalez-Reyes, A. Genetic dissection of a stem cell niche: the case of the Drosophila ovary. Dev. Dyn. 235, 2969–2979 (2006).
Kai, T., Williams, D. & Spradling, A. C. The expression profile of purified Drosophila germline stem cells. Dev. Biol. (2005).
Betschinger, J. & Knoblich, J. A. Dare to be different: asymmetric cell division in Drosophila, C. elegans and vertebrates. Curr. Biol. 14, R674–685 (2004).
Lee, T. & Luo, L. Mosaic analysis with a repressible cell marker for studies of gene function in neuronal morphogenesis. Neuron 22, 451–461 (1999).
Maurange, C., Cheng, L. & Gould, A. P. Temporal transcription factors and their targets schedule the end of neural proliferation in Drosophila. Cell 133, 891–902 (2008).
Clem, R. J., Fechheimer, M. & Miller, L. K. Prevention of apoptosis by a baculovirus gene during infection of insect cells. Science 254, 1388–1390 (1991).
Al-Hajj, M. & Clarke, M. F. Self-renewal and solid tumor stem cells. Oncogene 23, 7274–7282 (2004).
Bowman, S. K. et al. The tumor suppressors Brat and Numb regulate transit-amplifying neuroblast lineages in Drosophila. Dev. Cell 14, 535–546 (2008).
Bello, B., Reichert, H. & Hirth, F. The brain tumor gene negatively regulates neural progenitor cell proliferation in the larval central brain of Drosophila. Development 133, 2639–2648 (2006).
Betschinger, J., Mechtler, K. & Knoblich, J. A. Asymmetric segregation of the tumor suppressor brat regulates self-renewal in Drosophila neural stem cells. Cell 124, 1241–1253 (2006).
Lee, C. Y., Wilkinson, B. D., Siegrist, S. E., Wharton, R. P. & Doe, C. Q. Brat is a Miranda cargo protein that promotes neuronal differentiation and inhibits neuroblast self-renewal. Dev. Cell 10, 441–449 (2006).
Frank, D. J., Edgar, B. A. & Roth, M. B. The Drosophila melanogaster gene brain tumor negatively regulates cell growth and ribosomal RNA synthesis. Development 129, 399–407 (2002).
Page, S. L., McKim, K. S., Deneen, B., Van Hook, T. L. & Hawley, R. S. Genetic studies of mei-P26 reveal a link between the processes that control germ cell proliferation in both sexes and those that control meiotic exchange in Drosophila. Genetics 155, 1757–1772 (2000).
Komili, S., Farny, N. G., Roth, F. P. & Silver, P. A. Functional specificity among ribosomal proteins regulates gene expression. Cell 131, 557–571 (2007).
Knoblich, J., Jan, L. & Jan, Y. Asymmetric segregation of Numb and Prospero during cell division. Nature 377, 624–627 (1995).
Schroeder, T. Imaging stem-cell-driven regeneration in mammals. Nature 453, 345–351 (2008).
Bellaiche, Y., Gho, M., Kaltschmidt, J. A., Brand, A. H. & Schweisguth, F. Frizzled regulates localization of cell-fate determinants and mitotic spindle rotation during asymmetric cell division. Nature Cell Biol. 3, 50–57 (2001).
Gho, M., Bellaiche, Y. & Schweisguth, F. Revisiting the Drosophila microchaete lineage: a novel intrinsically asymmetric cell division generates a glial cell. Development 126, 3573–3584 (1999).
Worby, C. A., Simonson-Leff, N. & Dixon, J. E. RNA interference of gene expression (RNAi) in cultured Drosophila cells. Sci. STKE2001, PL1 (2001).
Hong, A., Lee-Kong, S., Iida, T., Sugimura, I. & Lilly, M. A. The p27cip/kip ortholog dacapo maintains the Drosophila oocyte in prophase of meiosis I. Development 130, 1235–1242 (2003).
Zhu, S. et al. Gradients of the Drosophila Chinmo BTB-zinc finger protein govern neuronal temporal identity. Cell 127, 409–422 (2006).
Ikeshima-Kataoka, H., Skeath, J. B., Nabeshima, Y.-i., Doe, C. Q. & Matsuzaki, F. Miranda directs Prospero to a daughter cell during Drosophila asymmetric divisions. Nature 390, 625–629 (1998).
González-Reyes, A., Elliott, H. & St Johnston, D. Polarization of both major body axes in Drosophila by gurken-torpedo signalling. Nature 375, 654–658 (1995).
Acknowledgements
We are especially grateful to Danièle Hernandez-Verdun, Bruno Bello, Frank Hirth, Andrew Vaughan and the Imaging facilities at IJM for experimental advice; to Danièle Hernandez-Verdun, Acaimo Gonzalez-Reyes, Antoine Guichet, Juliette Mathieu, Emily Richardson and Ralph Neumuller for critical reading. We are also grateful for reagents provided by Danièle Hernandez-Verdun, Joe Gall, Acaimo Gonzalez-Reyes, Fumio Matsuzaki, Christophe Antoniewski, Antoine Guichet, Christian Lehner, Alex Gould, Tzumin Lee, Barry Thompson, DHSB (Iowa University) and Bloomington Drosophila Stock center. We wish to thank Franck Pichaud (MRC-LMCB, UCL) in whose lab part of this work was performed. This work was funded by grants to J.R.H. (CNRS, ARC # 3802, and ANR # 06-JCJC-0092-01), P.F. (ARC post-doctoral fellowhip and EMBO long term fellowship), CM (CNRS), J.A.L. (CNRS, ARC# 3802) Ch.M. and Y.B. (CNRS, Curie).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Information (PDF 1684 kb)
Supplementary Information
Supplementary Movie 1 (AVI 10655 kb)
Supplementary Information
Supplementary Movie 2 (MOV 9381 kb)
Supplementary Information
Supplementary Movie 3 (AVI 10244 kb)
Supplementary Information
Supplementary Movie 4 (AVI 3223 kb)
Supplementary Information
Supplementary Movie 5 (AVI 2055 kb)
Supplementary Information
Supplementary Movie 6 (AVI 8579 kb)
Supplementary Information
Supplementary Movie 7 (AVI 5789 kb)
Supplementary Information
Supplementary Movie 8 (AVI 6369 kb)
Supplementary Information
Supplementary Movie 9 (AVI 17582 kb)
Supplementary Information
Supplementary Movie 10 (MOV 4591 kb)
Rights and permissions
About this article
Cite this article
Fichelson, P., Moch, C., Ivanovitch, K. et al. Live-imaging of single stem cells within their niche reveals that a U3snoRNP component segregates asymmetrically and is required for self-renewal in Drosophila. Nat Cell Biol 11, 685–693 (2009). https://doi.org/10.1038/ncb1874
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb1874
This article is cited by
-
Live imaging of stem cells in the germarium of the Drosophila ovary using a reusable gas-permeable imaging chamber
Nature Protocols (2018)
-
Mechanisms of protein homeostasis (proteostasis) maintain stem cell identity in mammalian pluripotent stem cells
Cellular and Molecular Life Sciences (2018)
-
Asymmetric cell division in plants: mechanisms of symmetry breaking and cell fate determination
Cellular and Molecular Life Sciences (2016)
-
Ribosome biogenesis dysfunction leads to p53-mediated apoptosis and goblet cell differentiation of mouse intestinal stem/progenitor cells
Cell Death & Differentiation (2015)
-
Microtubule-driven nuclear rotations promote meiotic chromosome dynamics
Nature Cell Biology (2015)