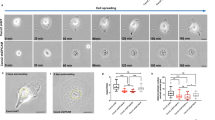
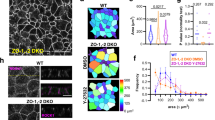
Abstract
Signalling by the GTPase RhoA, a key regulator of epithelial cell behaviour, can stimulate opposing processes: RhoA can promote junction formation and apical constriction, and reduce adhesion and cell spreading1,2. Molecular mechanisms are thus required that ensure spatially restricted and process-specific RhoA activation. For many fundamental processes, including assembly of the epithelial junctional complex, such mechanisms are still unknown. Here we show that p114RhoGEF is a junction-associated protein that drives RhoA signalling at the junctional complex and regulates tight-junction assembly and epithelial morphogenesis. p114RhoGEF is required for RhoA activation at cell–cell junctions, and its depletion stimulates non-junctional Rho signalling and induction of myosin phosphorylation along the basal domain. Depletion of GEF-H1, a RhoA activator inhibited by junctional recruitment3, does not reduce junction-associated RhoA activation. p114RhoGEF associates with a complex containing myosin II, Rock II and the junctional adaptor cingulin, indicating that p114RhoGEF is a component of a junction-associated Rho signalling module that drives spatially restricted activation of RhoA to regulate junction formation and epithelial morphogenesis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Terry, S., Nie, M., Matter, K. & Balda, M. S. Rho signaling and tight junction functions. Physiology (Bethesda) 25, 16–26 (2010).
Etienne-Manneville, S. & Hall, A. Rho GTPases in cell biology. Nature 420, 629–635 (2002).
Aijaz, S., D’Atri, F., Citi, S., Balda, M. S. & Matter, K. Binding of GEF-H1 to the tight junction-associated adaptor cingulin results in inhibition of Rho signaling and G1/S phase transition. Dev. Cell 8, 777–786 (2005).
Pertz, O. Spatio-temporal Rho GTPase signaling—where are we now? J. Cell Sci. 123, 1841–1850 (2010).
Heasman, S. J. & Ridley, A. J. Mammalian Rho GTPases: new insights into their functions from in vivo studies. Nat. Rev. Mol. Cell Biol. 9, 690–701 (2008).
Rossman, K. L., Der, C. J. & Sondek, J. GEF means go: turning on RHO GTPases with guanine nucleotide-exchange factors. Nat. Rev. Mol. Cell Biol. 6, 167–180 (2005).
Nusrat, A. et al. Rho protein regulates tight junctions and perijunctional actin organization in polarized epithelia. Proc. Natl Acad. Sci. USA 92, 10629–10633 (1995).
Braga, V. M., Machesky, L. M., Hall, A. & Hotchin, N. A. The small GTPases Rho and Rac are required for the establishment of cadherin-dependent cell–cell contacts. J. Cell Biol. 137, 1421–1431 (1997).
Noren, N. K., Niessen, C. M., Gumbiner, B. M. & Burridge, K. Cadherin engagement regulates Rho family GTPases. J. Biol. Chem. 276, 33305–33308 (2001).
Zondag, G. C. et al. Oncogenic Ras downregulates Rac activity, which leads to increased Rho activity and epithelial-mesenchymal transition. J. Cell Biol. 149, 775–782 (2000).
Coleman, M. L., Marshall, C. J. & Olson, M. F. RAS and RHO GTPases in G1-phase cell-cycle regulation. Nat. Rev. Mol. Cell Biol. 5, 355–366 (2004).
Ozdamar, B. et al. Regulation of the polarity protein Par6 by TGFbeta receptors controls epithelial cell plasticity. Science 307, 1603–1609 (2005).
Yamada, S. & Nelson, W. J. Localized zones of Rho and Rac activities drive initiation and expansion of epithelial cell–cell adhesion. J. Cell Biol. 178, 517–527 (2007).
Benais-Pont, G. et al. Identification of a tight junction-associated guanine nucleotide exchange factor that activates Rho and regulates paracellular permeability. J. Cell Biol. 160, 729–740 (2003).
Nie, M., Aijaz, S., Leefa Chong San, I. V., Balda, M. S. & Matter, K. The Y-box factor ZONAB/DbpA associates with GEF-H1/Lfc and mediates Rho-stimulated transcription. EMBO Rep. 10, 1125–1131 (2009).
Samarin, S. N., Ivanov, A. I., Flatau, G., Parkos, C. A. & Nusrat, A. Rho/Rho-associated kinase-II signaling mediates disassembly of epithelial apical junctions. Mol. Biol. Cell 18, 3429–3439 (2007).
Blomquist, A. et al. Identification and characterization of a novel Rho-specific guanine nucleotide exchange factor. Biochem. J. 352, 319–325 (2000).
Nagata, K. & Inagaki, M. Cytoskeletal modification of Rho guanine nucleotide exchange factor activity: identification of a Rho guanine nucleotide exchange factor as a binding partner for Sept9b, a mammalian septin. Oncogene 24, 65–76 (2005).
Niu, J., Profirovic, J., Pan, H., Vaiskunaite, R. & Voyno-Yasenetskaya, T. G Protein betagamma subunits stimulate p114RhoGEF, a guanine nucleotide exchange factor for RhoA and Rac1: regulation of cell shape and reactive oxygen species production. Circ. Res. 93, 848–856 (2003).
Matter, K. & Balda, M. S. Functional analysis of tight junctions. Methods 30, 228–234 (2003).
Birkenfeld, J., Nalbant, P., Yoon, S. H. & Bokoch, G. M. Cellular functions of GEF-H1, a microtubule-regulated Rho-GEF: is altered GEF-H1 activity a crucial determinant of disease pathogenesis? Trends Cell Biol. 18, 210–219 (2008).
Kakiashvili, E. et al. GEF-H1 mediates tumor necrosis factor-alpha-induced Rho activation and myosin phosphorylation: role in the regulation of tubular paracellular permeability. J. Biol. Chem. 284, 11454–11466 (2009).
Zegers, M. M., O’Brien, L. E., Yu, W., Datta, A. & Mostov, K. E. Epithelial polarity and tubulogenesis in vitro. Trends Cell Biol. 13, 169–176 (2003).
Yoshizaki, H. et al. Activity of Rho-family GTPases during cell division as visualized with FRET-based probes. J. Cell Biol. 162, 223–232 (2003).
Sterpetti, P. et al. Activation of the Lbc Rho exchange factor proto-oncogene by truncation of an extended C terminus that regulates transformation and targeting. Mol. Cell Biol. 19, 1334–1345 (1999).
Krendel, M., Zenke, F. T. & Bokoch, G. M. Nucleotide exchange factor GEF-H1 mediates cross-talk between microtubules and the actin cytoskeleton. Nat. Cell Biol. 4, 294–301 (2002).
Cordenonsi, M. et al. Cingulin contains globular and coiled-coil domains and interacts with ZO-1, ZO-2, ZO-3, and myosin. J. Cell Biol. 147, 1569–1582 (1999).
Steed, E., Balda, M. S. & Matter, K. Dynamics and functions of tight junctions. Trends Cell Biol. 20, 142–149 (2010).
Guillemot, L. & Citi, S. Cingulin regulates claudin-2 expression and cell proliferation through the small GTPase RhoA. Mol. Biol. Cell 17, 3569–3577 (2006).
Braga, V. M. Cell–cell adhesion and signalling. Curr. Opin. Cell Biol. 14, 546–556 (2002).
Noren, N. K., Arthur, W. T. & Burridge, K. Cadherin engagement inhibits RhoA via p190RhoGAP. J. Biol. Chem. 278, 13615–13618 (2003).
Abouhamed, M. et al. Myosin IXa regulates epithelial differentiation and its deficiency results in hydrocephalus. Mol. Biol. Cell 20, 5074–5084 (2009).
Matter, K., McDowell, W., Schwartz, R. T. & Hauri, H. P. Asynchronous transport to the cell surface of intestinal brush border hydrolases is not due to differential trimming of N-linked oligosaccharides. J. Biol. Chem. 264, 13131–13139 (1989).
Osler, M. E., Chang, M. S. & Bader, D. M. Bves modulates epithelial integrity through an interaction at the tight junction. J. Cell Sci. 118, 4667–4678 (2005).
Sourisseau, T. et al. Regulation of PCNA and cyclin D1 expression and epithelial morphogenesis by the ZO-1-regulated transcription factor ZONAB/DbpA. Mol. Cell Biol. 26, 2387–2398 (2006).
Jaffe, A. B., Kaji, N., Durgan, J. & Hall, A. Cdc42 controls spindle orientation to position the apical surface during epithelial morphogenesis. J. Cell Biol. 183, 625–633 (2008).
Steed, E., Rodrigues, N. T., Balda, M. S. & Matter, K. Identification of MarvelD3 as a tight junction-associated transmembrane protein of the occludin family. BMC Cell Biol. 10, 95 (2009).
Balda, M. S. et al. Functional dissociation of paracellular permeability and transepithelial electrical resistance and disruption of the apical-basolateral intramembrane diffusion barrier by expression of a mutant tight junction membrane protein. J. Cell Biol. 134, 1031–1049 (1996).
Acknowledgements
S.J.T. is supported by a Fight for Sight Studentship. This research was supported by Fight for Sight and the Wellcome Trust.
Author information
Authors and Affiliations
Contributions
S.J.T. carried out most of the experiments. All other authors carried out particular subsets of experiments. S.J.T., M.S.B. and K.M. designed the project and wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Information (PDF 1374 kb)
Rights and permissions
About this article
Cite this article
Terry, S., Zihni, C., Elbediwy, A. et al. Spatially restricted activation of RhoA signalling at epithelial junctions by p114RhoGEF drives junction formation and morphogenesis. Nat Cell Biol 13, 159–166 (2011). https://doi.org/10.1038/ncb2156
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb2156
This article is cited by
-
Patterning of the cell cortex by Rho GTPases
Nature Reviews Molecular Cell Biology (2024)
-
Targeting non-muscle myosin II promotes corneal endothelial migration through regulating lamellipodial dynamics
Journal of Molecular Medicine (2019)
-
cAMP guided his way: a life for G protein-mediated signal transduction and molecular pharmacology—tribute to Karl H. Jakobs
Naunyn-Schmiedeberg's Archives of Pharmacology (2019)
-
The mechanobiology of tight junctions
Biophysical Reviews (2019)
-
An apical MRCK-driven morphogenetic pathway controls epithelial polarity
Nature Cell Biology (2017)